The Cerebellum Is a Key Structure in the Neural Network for Mentalizing: An MRI Study in the Behavioral Variant of Frontotemporal Dementia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
- (1)
- Disinhibition (socially inappropriate behaviour, loss of decorum, and impulsiveness)
- (2)
- Apathy or inertia (quantitative reduction in purposeful voluntary behaviours)
- (3)
- Loss of empathy
- (4)
- Repetitive behaviours, ritualisms, or stereotypes
- (5)
- Hyperorality (oral exploration of objects, substantial changes in food preferences, binge eating, and increased consumption of tobacco or alcohol)
- (6)
- Cognitive modifications (deficit of executive functions with at least partial preservation of episodic memory and visuospatial skills).
2.2. MRI Acquisition Protocol
2.3. Image Processing and Data Analysis
2.3.1. Voxel-Based Morphometry
2.3.2. Resting-State fMRI Data Preprocessing
2.4. Definition of Regions of Interest (ROIs) and Seed-Based Analyses
2.5. ToM Assessment and Analysis
2.6. Behavioural Correlations with Functional Connectivity
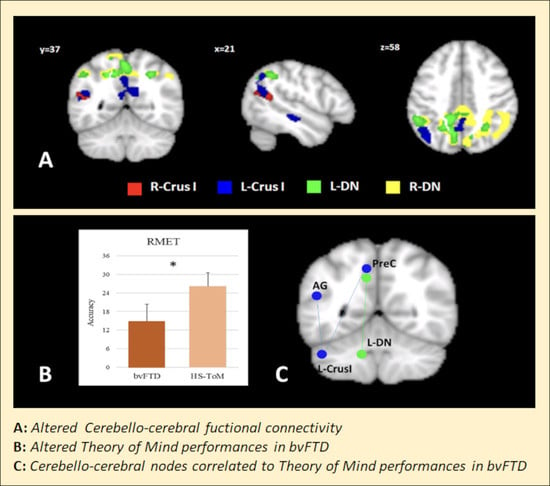
3. Results
3.1. Voxel-Based Morphometry
3.2. Seed-Based FC Results
3.3. ToM Assessment and Correlation with Cerebello-Cerebral FC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmahmann, J.D.; Sherman, J.C. The Cerebellar Cognitive Affective Syndrome. Brain 1998, 121, 561–579. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Weilburg, J.B.; Sherman, J.C. The Neuropsychiatry of the Cerebellum—Insights from the Clinic. Cerebellum 2007, 6, 254–267. [Google Scholar] [CrossRef]
- Mariën, P.; Beaton, A. The Enigmatic Linguistic Cerebellum: Clinical Relevance and Unanswered Questions on Nonmotor Speech and Language Deficits in Cerebellar Disorders. Cerebellum Ataxias 2014, 1, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brissenden, J.A.; Levin, E.J.; Osher, D.E.; Halko, M.A.; Somers, D.C. Functional Evidence for a Cerebellar Node of the Dorsal Attention Network. J. Neurosci. 2016, 36, 6083–6096. [Google Scholar] [CrossRef] [Green Version]
- Lupo, M.; Olivito, G.; Iacobacci, C.; Clausi, S.; Romano, S.; Masciullo, M.; Molinari, M.; Cercignani, M.; Bozzali, M.; Leggio, M. The Cerebellar Topography of Attention Sub-Components in Spinocerebellar Ataxia Type 2. Cortex 2018, 108, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D. The Cerebellum and Cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef]
- Johnen, A.; Bertoux, M. Psychological and Cognitive Markers of Behavioral Variant Frontotemporal Dementia—A Clinical Neuropsychologist’s View on Diagnostic Criteria and Beyond. Front. Neurol. 2019, 10, 594. [Google Scholar] [CrossRef] [Green Version]
- Frith, C.D.; Frith, U. The Neural Basis of Mentalizing. Neuron 2006, 50, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Amodio, D.M.; Frith, C.D. Meeting of Minds: The Medial Frontal Cortex and Social Cognition. Nat. Rev. Neurosci. 2006, 7, 268–277. [Google Scholar] [CrossRef]
- Saxe, R.; Kanwisher, N. People Thinking about Thinking People: The Role of the Temporo-Parietal Junction in “Theory of Mind”. Neuroimage 2003, 19, 1835–1842. [Google Scholar] [CrossRef]
- Aichhorn, M.; Perner, J.; Weiss, B.; Kronbichler, M.; Staffen, W.; Ladurner, G. Temporo-parietal junction activity in theory-of-mind tasks: Falseness, beliefs, or attention. J. Cogn. Neurosci. 2009, 21, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Van Overwalle, F.; Mariën, P. Functional Connectivity between the Cerebrum and Cerebellum in Social Cognition: A Multi-Study Analysis. Neuroimage 2016, 124, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Adolphs, R. Processing of Emotional and Social Information by the Huamn Amygdala. In The Cognitive Neuroscience; MIT Press: Cambridge, MA, USA, 2004; pp. 1017–1030. [Google Scholar]
- Kipps, C.M.; Duggins, A.J.; McCusker, E.A.; Calder, A.J. Disgust and Happiness Recognition Correlate with Anteroventral Insula and Amygdala Volume Respectively in Preclinical Huntington’s Disease. J. Cogn. Neurosci. 2007, 19, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Gao, Z.; Wang, X.; Liu, X.; Knight, R.T.; Hof, P.R.; Fan, J. Anterior Insular Cortex Is Necessary for Empathetic Pain Perception. Brain 2012, 135, 2726–2735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnstone, T.; van Reekum, C.M.; Oakes, T.R.; Davidson, R.J. The Voice of Emotion: An FMRI Study of Neural Responses to Angry and Happy Vocal Expressions. Soc. Cogn. Affect. Neurosci. 2006, 1, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Buckner, R.L.; Krienen, F.M.; Castellanos, A.; Diaz, J.C.; Thomas Yeo, B.T. The Organization of the Human Cerebellum Estimated by Intrinsic Functional Connectivity. J. Neurophysiol. 2011, 106, 2322–2345. [Google Scholar] [CrossRef] [Green Version]
- Habas, C.; Kamdar, N.; Nguyen, D.; Prater, K.; Beckmann, C.F.; Menon, V.; Greicius, M.D. Distinct Cerebellar Contributions to Intrinsic Connectivity Networks. J. Neurosci. 2009, 29, 8586–8594. [Google Scholar] [CrossRef]
- Van Overwalle, F.; D’aes, T.; Mariën, P. Social Cognition and the Cerebellum: A Meta-Analytic Connectivity Analysis. Hum. Brain Mapp. 2015, 36, 5137–5154. [Google Scholar] [CrossRef]
- Clausi, S.; Olivito, G.; Siciliano, L.; Lupo, M.; Bozzali, M.; Masciullo, M.; Molinari, M.; Romano, S.; Leggio, M. The Neurobiological Underpinning of the Social Cognition Impairments in Patients with Spinocerebellar Ataxia Type 2. Cortex 2021, 138, 101–112. [Google Scholar] [CrossRef]
- Lupo, M.; Olivito, G.; Clausi, S.; Siciliano, L.; Riso, V.; Bozzali, M.; Santorelli, F.M.; Silvestri, G.; Leggio, M. Cerebello-Cortical Alterations Linked to Cognitive and Social Problems in Patients with Spastic Paraplegia Type 7: A Preliminary Study. Front. Neurol. 2020, 11, 82. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, A.A. The Cerebellum in Social Cognition. Front. Cell. Neurosci. 2018, 12, 145. [Google Scholar] [CrossRef] [Green Version]
- Clausi, S.; Olivito, G.; Siciliano, L.; Lupo, M.; Laghi, F.; Baiocco, R.; Leggio, M. The Cerebellum Is Linked to Theory of Mind Alterations in Autism. A Direct Clinical and MRI Comparison between Individuals with Autism and Cerebellar Neurodegenerative Pathologies. Autism Res. 2021, 14, 2300–2313. [Google Scholar] [CrossRef] [PubMed]
- Bertoux, M.; de Souza Lc, L.C.; O’Callaghan, C.; Greve, A.; Sarazin, M.; Dubois, B.; Hornberger, M. Social Cognition Deficits: The Key to Discriminate Behavioral Variant Frontotemporal Dementia from Alzheimer’s Disease Regardless of Amnesia? J. Alzheimer’s Dis. 2015, 49, 1065–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibanez, A.; Manes, F. Contextual Social Cognition and the Behavioral Variant of Frontotemporal Dementia. Neurology 2012, 78, 1354–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron-Cohen, S.; Wheelwright, S.; Hill, J.; Raste, Y.; Plumb, I. The “Reading the Mind in the Eyes” Test Revised Version: A Study with Normal Adults, and Adults with Asperger Syndrome or High-Functioning Autism. J. Child Psychol. Psychiatry 2001, 42, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, M.L.; Pawelke, S.; Bisenius, S.; Kynast, J.; Schuemberg, K.; Polyakova, M.; Anderl-Straub, S.; Danek, A.; Fassbender, K.; Jahn, H.; et al. A Modified Reading the Mind in the Eyes Test Predicts Behavioral Variant Frontotemporal Dementia Better than Executive Function Tests. Front. Aging Neurosci. 2018, 10, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, J.A. Don’t Forget the Little Brain: A Framework for Incorporating the Cerebellum into the Understanding of Cognitive Aging. Neurosci. Biobehav. Rev. 2022, 137, 104639. [Google Scholar] [CrossRef]
- Guo, C.C.; Tan, R.; Hodges, J.R.; Hu, X.; Sami, S.; Hornberger, M. Network-Selective Vulnerability of the Human Cerebellum to Alzheimer’s Disease and Frontotemporal Dementia. Brain 2016, 139, 1527–1538. [Google Scholar] [CrossRef]
- Brun, A. Frontal Lobe Degeneration of Non-Alzheimer Type Revisited. Dementia 1993, 4, 126–131. [Google Scholar] [CrossRef]
- Thomas Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The Organization of the Human Cerebral Cortex Estimated by Intrinsic Functional Connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef]
- Chen, Y.; Kumfor, F.; Landin-Romero, R.; Irish, M.; Hodges, J.R.; Piguet, O. Cerebellar Atrophy and Its Contribution to Cognition in Frontotemporal Dementias. Ann. Neurol. 2018, 84, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Van Overwalle, F.; van de Steen, F.; Mariën, P. Dynamic Causal Modeling of the Effective Connectivity between the Cerebrum and Cerebellum in Social Mentalizing across Five Studies. Cogn. Affect. Behav. Neurosci. 2019, 19, 211–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerami, C.; Dodich, A.; Canessa, N.; Crespi, C.; Marcone, A.; Cortese, F.; Chierchia, G.; Scola, E.; Falini, A.; Cappa, S.F. Neural Correlates of Empathic Impairment in the Behavioral Variant of Frontotemporal Dementia. Alzheimer’s Dement. 2014, 10, 827–834. [Google Scholar] [CrossRef]
- Diehl-Schmid, J.; Pohl, C.; Ruprecht, C.; Wagenpfeil, S.; Foerstl, H.; Kurz, A. The Ekman 60 Faces Test as a Diagnostic Instrument in Frontotemporal Dementia. Arch. Clin. Neuropsychol. 2007, 22, 459–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.P.; Onyike, C.U.; et al. Sensitivity of Revised Diagnostic Criteria for the Behavioural Variant of Frontotemporal Dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-Mental State. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Alberici, A.; Geroldi, C.; Cotelli, M.; Adorni, A.; Calabria, M.; Rossi, G.; Borroni, B.; Padovani, A.; Zanetti, O.; Kertesz, A. The Frontal Behavioural Inventory (Italian Version) Differentiates Frontotemporal Lobar Degeneration Variants from Alzheimer’s Disease. Neurol. Sci. 2007, 28, 80–86. [Google Scholar] [CrossRef]
- Berg, G.; Edwards, D.F.; Danzinger, W.L.; Berg, L. Longitudinal Change in Three Brief Assessments of SDAT. J. Am. Geriatr. Soc. 1987, 35, 205–212. [Google Scholar] [CrossRef]
- Carlesimo, G.A.; Caltagirone, C.; Gainotti, G. The Mental Deterioration Battery: Normative Data, Diagnostic Reliability and Qualitative Analyses of Cognitive Impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur. Neurol. 1996, 36, 378–384. [Google Scholar] [CrossRef]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. Rey-Osterrieth Complex Figure: Normative Values in an Italian Population Sample. Neurol. Sci. 2002, 22, 443–447. [Google Scholar] [CrossRef]
- Monaco, M.; Costa, A.; Caltagirone, C.; Carlesimo, G.A. Forward and Backward Span for Verbal and Visuo-Spatial Data: Standardization and Normative Data from an Italian Adult Population. Neurol. Sci. 2013, 34, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.C. Progressive Matrices. Set A, Ab, B. Board and Book Form; H.K. Lewis: London, UK, 1947. [Google Scholar]
- Quaranta, D.; Caprara, A.; Piccininni, C.; Vita, M.G.; Gainotti, G.; Marra, C. Standardization, Clinical Validation, and Typicality Norms of a New Test Assessing Semantic Verbal Fluency. Arch. Clin. Neuropsychol. 2016, 31, 434–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marra, C.; Gainotti, G.; Scaricamazza, E.; Piccininni, C.; Ferraccioli, M.; Quaranta, D. The Multiple Features Target Cancellation (MFTC): An Attentional Visual Conjunction Search Test. Normative Values for the Italian Population. Neurol. Sci. 2013, 34, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, A.; Venneri, A. Una Versione Abbreviata Del Test Di Stroop: Dati Normativi Nella Popolazione Italiana. Nuova Riv. Neurol. 2002, 12, 111–115. [Google Scholar]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Venneri, A. Modified Card Sorting Test: Normative Data. J. Clin. Exp. Neuropsychol. 2010, 26, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Giovagnoli, A.R.; Del Pesce, M.; Mascheroni, S.; Simoncelli, M.; Laiacona, M.; Capitani, E. Trail Making Test: Normative Values from 287 Normal Adult Controls. Ital. J. Neurol. Sci. 1996, 17, 305–309. [Google Scholar] [CrossRef]
- Diedrichsen, J.; Balsters, J.H.; Flavell, J.; Cussans, E.; Ramnani, N. A Probabilistic MR Atlas of the Human Cerebellum. Neuroimage 2009, 46, 39–46. [Google Scholar] [CrossRef]
- Olivito, G.; Serra, L.; Marra, C.; di Domenico, C.; Caltagirone, C.; Toniolo, S.; Cercignani, M.; Leggio, M.; Bozzali, M. Cerebellar Dentate Nucleus Functional Connectivity with Cerebral Cortex in Alzheimer’s Disease and Memory: A Seed-Based Approach. Neurobiol. Aging 2020, 89, 32–40. [Google Scholar] [CrossRef]
- Dum, R.P.; Strick, P.L. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003, 89, 634–639. [Google Scholar] [CrossRef]
- Serafin, M.; Surian, L. Il Test Degli Occhi: Uno Strumento per Valutare La “Teoria Della Mente”. Giornale Italiano di Psicologia 2004, 31, 839–862. [Google Scholar]
- Van Overwalle, F.; Baetens, K.; Mariën, P.; Vandekerckhove, M. Social Cognition and the Cerebellum: A Meta-Analysis of over 350 FMRI Studies. Neuroimage 2014, 86, 554–572. [Google Scholar] [CrossRef] [PubMed]
- Van Overwalle, F.; Pu, M.; Ma, Q.; Li, M.; Haihambo, N.; Baetens, K.; Deroost, N.; Baeken, C.; Heleven, E. The Involvement of the Posterior Cerebellum in Reconstructing and Predicting Social Action Sequences. Cerebellum 2022, 21, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; Macleod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A Default Mode of Brain Function Source: Proceedings of the National Academy of Sciences of the United States of America, Vol. Published by: National Academy of Sciences Stable URL: Http://Www.Jstor.Org/Stable/3054743 REFERENCES Linked References Are. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schilbach, L.; Eickhoff, S.B.; Rotarska-Jagiela, A.; Fink, G.R.; Vogeley, K. Minds at Rest? Social Cognition as the Default Mode of Cognizing and Its Putative Relationship to the “Default System” of the Brain. Conscious Cogn. 2008, 17, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Krienen, F.M.; Buckner, R.L. Segregated Fronto-Cerebellar Circuits Revealed by Intrinsic Functional Connectivity. Cereb. Cortex 2009, 19, 2485–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, J.A.; Seidler, R.D.; Hassevoort, K.M.; Benson, B.L.; Welsh, R.C.; Lee Wiggins, J.; Jaeggi, S.M.; Buschkuehl, M.; Monk, C.S.; Jonides, J.; et al. Resting State Cortico-Cerebellar Functional Connectivity Networks: A Comparison of Anatomical and Self-Organizing Map Approaches. Front. Neuroanat. 2012, 6, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, F.A.; Strick, P.L. Basal Ganglia and Cerebellar Loops: Motor and Cognitive Circuits. Brain Res. Rev. 2000, 31, 236–250. [Google Scholar] [CrossRef]
- Allen, G.; McColl, R.; Barnard, H.; Ringe, W.K.; Fleckenstein, J.; Cullum, C.M. Magnetic Resonance Imaging of Cerebellar-Prefrontal and Cerebellar-Parietal Functional Connectivity. Neuroimage 2005, 28, 39–48. [Google Scholar] [CrossRef]
- Henry, J.D.; Phillips, L.H.; von Hippel, C. A Meta-Analytic Review of Theory of Mind Difficulties in Behavioural-Variant Frontotemporal Dementia. Neuropsychologia 2014, 56, 53–62. [Google Scholar] [CrossRef]
- Adenzato, M.; Poletti, M. Theory of Mind Abilities in Neurodegenerative Diseases: An Update and a Call to Introduce Mentalizing Tasks in Standard Neuropsychological Assessments. Clin. Neuropsychiatry 2013, 10, 226–234. [Google Scholar]
- Gregory, C.; Lough, S.; Stone, V.; Erzinclioglu, S.; Martin, L.; Baron-Cohen, S.; Hodges, J.R. Theory of Mind in Patients with Frontal Variant Frontotemporal Dementia and Alzheimer’s Disease: Theoretical and Practical Implications. Brain 2002, 125, 752–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torralva, T.; Roca, M.; Gleichgerrcht, E.; Bekinschtein, T.; Manes, F. A Neuropsychological Battery to Detect Specific Executive and Social Cognitive Impairments in Early Frontotemporal Dementia. Brain 2009, 132, 1299–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molenberghs, P.; Cunnington, R.; Mattingley, J.B. Brain Regions with Mirror Properties: A Meta-Analysis of 125 Human FMRI Studies. Neurosci. Biobehav. Rev. 2012, 36, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Olson, I.R. The Original Social Network: White Matter and Social Cognition. Trends Cogn. Sci. 2018, 22, 504–516. [Google Scholar] [CrossRef]
- Gellersen, H.M.; Guo, C.C.; O’callaghan, C.; Tan, R.H.; Sami, S.; Hornberger, M. Cerebellar Atrophy in Neurodegeneration—A Meta-Analysis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 780–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenna, M.C.; Chipika, R.H.; Li Hi Shing, S.; Christidi, F.; Lope, J.; Doherty, M.A.; Hengeveld, J.C.; Vajda, A.; McLaughlin, R.L.; Hardiman, O.; et al. Infratentorial Pathology in Frontotemporal Dementia: Cerebellar Grey and White Matter Alterations in FTD Phenotypes. J. Neurol. 2021, 268, 4687–4697. [Google Scholar] [CrossRef]
- Ramnani, N. The Primate Cortico-Cerebellar System: Anatomy and Function. Nat. Rev. Neurosci. 2006, 7, 511–522. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Pandya, D.N. Prefrontal Cortex Projections to the Basilar Pons in Rhesus Monkey: Implications for the Cerebellar Contribution to Higher Function. Neurosci. Lett. 1995, 199, 175–178. [Google Scholar] [CrossRef]
- Oulad Ben Taib, N.; Manto, M. Reinstating the Ability of the Motor Cortex to Modulate Cutaneomuscular Reflexes in Hemicerebellectomized Rats. Brain Res. 2008, 1204, 59–68. [Google Scholar] [CrossRef]
- Olivito, G.; Dayan, M.; Battistoni, V.; Clausi, S.; Cercignani, M.; Molinari, M.; Leggio, M.; Bozzali, M. Bilateral Effects of Unilateral Cerebellar Lesions as Detected by Voxel Based Morphometry and Diffusion Imaging. PLoS ONE 2017, 12, e180439. [Google Scholar] [CrossRef] [Green Version]
- Stoodley, C.J.; Schmahmann, J.D. Functional Topography in the Human Cerebellum: A Meta-Analysis of Neuroimaging Studies. Neuroimage 2009, 44, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Baillieux, H.; de Smet, H.J.; Dobbeleir, A.; Paquier, P.F.; de Deyn, P.P.; Mariën, P. Cognitive and Affective Disturbances Following Focal Cerebellar Damage in Adults: A Neuropsychological and SPECT Study. Cortex 2010, 46, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Groen, W.B.; Buitelaar, J.K.; van der Gaag, R.J.; Zwiers, M.P. Pervasive Microstructural Abnormalities in Autism: A DTI Study. J. Psychiatry Neurosci. 2011, 36, 32–40. [Google Scholar] [CrossRef]
- Friston, K.J.; Frith, C.D.; Liddle, P.F.; Frackowiak, R.S.J. Functional Connectivity: The Principal-Component Analysis of Large (PET) Data Sets. J. Cereb. Blood Flow Metab. 1993, 13, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Leggio, M.; Molinari, M. Cerebellar Sequencing: A Trick for Predicting the Future. Cerebellum 2015, 14, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Molinari, M.; Restuccia, D.; Leggio, M.G. State Estimation, Response Prediction, and Cerebellar Sensory Processing for Behavioral Control. Cerebellum 2009, 8, 399–402. [Google Scholar] [CrossRef]
- Brown, E.C.; Brüne, M. The Role of Prediction in Social Neuroscience. Front. Hum. Neurosci. 2012, 6, 147. [Google Scholar] [CrossRef]





| bvFTD | HS-MRI | HDS-ToM | |||||
|---|---|---|---|---|---|---|---|
| Sociodemographical and Clinical Variables | M | SD | M | SD | M | SD | |
| Age (months) | 69.8 | 5.63 | 69.1 | 16.6 | 67.2 | 5.9 | |
| Education (years) | 11.1 | 4.82 | 12.9 | 6.7 | 13.5 | 3.4 | |
| Sex (M/F) | 11/4 (73%/27%) | 17/17(50%/50%) | 15/12 (55%44%) | ||||
| Illness duration (months) | 34.1 | 16.06 | |||||
| Clinical Dementia Rating scale (CDR) [39] | 1.1 | 0.56 | |||||
| Neuropsychological assessment | Cut-off | M | SD | ||||
| Mini Mental State Examination [37] | >23.80 | 24.9 | 4.67 | ||||
| RAVLT: immediate recall * [40] | >28.53 | 26.8 | 8.85 | ||||
| RAVLT: delayed recall * [40] | >4.69 | 3.3 | 2.31 | ||||
| RAVLT: recognition accuracy * [40] | >0.88 | 0.7 | 0.15 | ||||
| Rey-Osterrieth figure copy * [41] | >28.87 | 23.6 | 9.48 | ||||
| Rey-Osterrieth figure recall * [41] | >9.46 | 6.9 | 4.39 | ||||
| Digit span forward [42] | >4.26 | 5.1 | 1.13 | ||||
| Digit span backward [42] | >2.65 | 3.1 | 0.99 | ||||
| Corsi’s test forward [42] | >3.46 | 3.9 | 1.51 | ||||
| Corsi’s test backward [42] | >3.08 | 3.3 | 1.28 | ||||
| Raven’s Progressive Colored Matrices [43] | >18.96 | 18.9 | 8.47 | ||||
| Copy of figures [40] | >7.18 | 9.1 | 3.05 | ||||
| Copy of figures with landmarks * [40] | >61.85 | 56.3 | 24.35 | ||||
| Phonological Verbal Fluency [44] | >17.35 | 19.1 | 7.66 | ||||
| Semantic Verbal Fluency [44] | >9.28 | 10.5 | 6.48 | ||||
| MFTC accuracy [45] | >0.869 | 0.8 | 0.16 | ||||
| MFTC false alarms [45] | <2.77 | 2.2 | 5.09 | ||||
| MFTC time of execution [45] | <135.73 | 78.3 | 47.87 | ||||
| Stroop’s test: interference time * [46] | <36.92 | 67.7 | 54.91 | ||||
| Stroop’s test: interference errors [46] | <4.24 | 3.5 | 3.29 | ||||
| MWCST: categories [47] | >2 | 3.0 | 1.93 | ||||
| MWCST: perseverative errors * [47] | <6.41 | 9.6 | 11.47 | ||||
| Trail Making part A * [48] | <93 | 99.5 | 44.53 | ||||
| Trail Making part B * [48] | <282 | 302.0 | 138.19 | ||||
| Trail Making B-A * [48] | <186 | 202.5 | 100.54 | ||||
| Behavioral assessment | |||||||
| FBI: Apathy [38] | 18.3 | 5.96 | |||||
| FBI: Disinhibition [38] | 11.5 | 6.68 | |||||
| FBI: Total Score * [38] | <28.6 | 29.8 | 11.06 | ||||
| Regions | Size | Side | MNI Coordinates (mm) | Peak Z-Scores | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Lobule I–IV | 3568 | R | 9 | −35 | −13 | 5.25 |
| Lobule V | R | 23 | −31 | −22 | 4.78 | |
| 14 | −45 | −8 | 4.71 | |||
| Crus I | 4455 | R | 46 | −47 | −36 | 4.07 |
| R | 42 | −61 | −21 | 3.87 | ||
| R | 42 | −62 | −29 | 3.81 | ||
| Lobule VI | 4770 | L | −40 | −46 | −30 | 4.00 |
| Crus I | L | −44 | −48 | −38 | 3.86 | |
| L | −44 | −58 | −36 | 3.62 | ||
| Cerebellar Regions | Cerebral Regions | Size (NoV) | Side | MNI Coordinates (mm) | Peak Z-Scores | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| R-CrusI | Angular gyrus | 279 | L | −48 | −52 | −18 | 4.45 |
| L | −54 | −58 | 22 | 4.13 | |||
| L-Crus I | Parahippocampal gyrus | 267 | L | −30 | −32 | −14 | 4.71 |
| L | −30 | −38 | −6 | 4.14 | |||
| Lateral occipital cortex | 445 | L | −42 | −68 | 42 | 4.03 | |
| Angular gyrus | L | −44 | −50 | 22 | 3.01 | ||
| Lateral occipital cortex | L | −48 | −60 | 42 | 3.77 | ||
| Middle temporal gyrus | 190 | L | −60 | −8 | −22 | 3.96 | |
| L | −52 | −24 | −8 | 3.86 | |||
| L | −52 | −18 | −14 | 3.51 | |||
| Precuneus | 494 | R | 0 | −48 | 38 | 3.86 | |
| R | 12 | −54 | 24 | 3.73 | |||
| L | −4 | −60 | 20 | 3.72 | |||
| L-DN | Precuneus | 1851 | L | −12 | −60 | 48 | 5.39 |
| L | −4 | −62 | 62 | 5.20 | |||
| L | −12 | −46 | 42 | 4.80 | |||
| Supramarginal gyrus | 251 | L | −54 | −46 | 40 | 4.01 | |
| Lateral occipital cortex | L | −44 | −64 | 44 | 3.00 | ||
| R-DN | Precuneus | 3453 | R | 8 | −78 | 48 | 6.02 |
| L | −12 | −60 | 48 | 5.51 | |||
| Lateral occipital cortex | R | 18 | −72 | 48 | 5.27 | ||
| Supramarginal gyrus | 323 | L | −46 | −46 | 44 | 4.46 | |
| Lateral occipital cortex | L | −44 | −62 | 44 | 4.39 | ||
| Angular gyrus | L | −50 | −54 | 46 | 4.09 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivito, G.; Quaranta, D.; Siciliano, L.; Caraglia, N.; Caprara, A.; Marra, C.; Leggio, M.; Silveri, M.C. The Cerebellum Is a Key Structure in the Neural Network for Mentalizing: An MRI Study in the Behavioral Variant of Frontotemporal Dementia. Biomedicines 2022, 10, 2901. https://doi.org/10.3390/biomedicines10112901
Olivito G, Quaranta D, Siciliano L, Caraglia N, Caprara A, Marra C, Leggio M, Silveri MC. The Cerebellum Is a Key Structure in the Neural Network for Mentalizing: An MRI Study in the Behavioral Variant of Frontotemporal Dementia. Biomedicines. 2022; 10(11):2901. https://doi.org/10.3390/biomedicines10112901
Chicago/Turabian StyleOlivito, Giusy, Davide Quaranta, Libera Siciliano, Naike Caraglia, Alessia Caprara, Camillo Marra, Maria Leggio, and Maria Caterina Silveri. 2022. "The Cerebellum Is a Key Structure in the Neural Network for Mentalizing: An MRI Study in the Behavioral Variant of Frontotemporal Dementia" Biomedicines 10, no. 11: 2901. https://doi.org/10.3390/biomedicines10112901