Novel Chromone-Containing Allylmorpholines Induce Anxiolytic-like and Sedative Effects in Adult Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Drugs
2.2. Experimental Design and Behavioral Testing
2.3. Sequence Identity Analysis
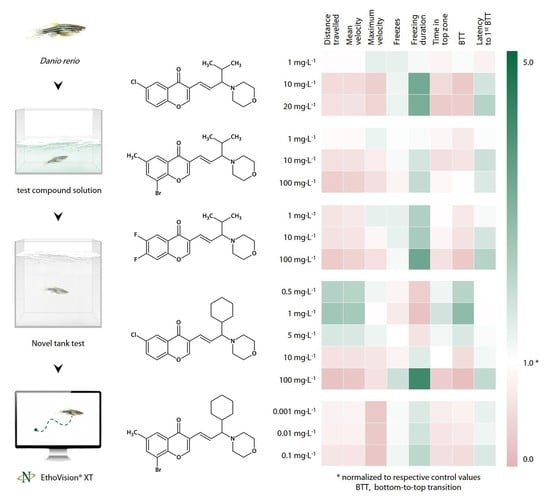
3. Results
3.1. CCAM Screening
3.1.1. Sedative Activity of Compound 1 (9a) in the Novel Tank Test
3.1.2. Sedative Activity of Compound 2 (9j) in the Novel Tank Test
3.1.3. Sedative Activity of Compound 3 (9l) in the Novel Tank Test
3.1.4. Sedative Activity of Compound 4 (33a) in the Novel Tank Test
3.1.5. Sedative Effect of Compound 5 (33b) in the Novel Tank Test
3.2. Putative Antagonist Testing
3.2.1. Effects of Compound 1 (9a) Following Equimolar N-Methyl-D-Aspartate Pre-Exposure in the Novel Tank Test
3.2.2. Effects of N-Methyl-D-Aspartate in the Novel Tank Test
3.2.3. Sedative Effect of Quinolinic Acid in the Novel Tank Test
3.2.4. Effects of Compound 1 (9a) Following Equimolar Biperiden Pre-Exposure in the Novel Tank Test
3.3. Anxiolytic-like Activity of Compound 33a
3.3.1. Anxiolytic-like Effect of Compound 4 (33a) in the Novel Tank Test
3.3.2. Anxiolytic-like Effect of Compound 4 (33a) in the Light/Dark Box Test
4. Discussion
4.1. CCAM Screening
4.2. Contribution of Glutamatergic and Cholinergic Mechanisms to the Effects of the CCAM
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanaka, M.; Vécsei, L. Editorial of Special Issue ‘Dissecting Neurological and Neuropsychiatric Diseases: Neurodegeneration and Neuroprotection’. Int. J. Mol. Sci. 2022, 23, 6991. [Google Scholar] [CrossRef] [PubMed]
- Pal’chikov, V.A. Morpholines. Synthesis and Biological Activity. Rus. J. Org. Chem. 2013, 49, 787–814. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R.K. Morpholine as ubiquitous pharmacophore in medicinal chemistry: Deep insight into the structure-activity relationship (SAR). Bioorg. Chem. 2020, 96, 103578. [Google Scholar] [CrossRef]
- Yan, Y.-M.; Meng, X.-H.; Bai, F.-H.; Cheng, Y.-X. Nonpeptide small molecules with a ten-membered macrolactam or a morpholine motif from the insect American cockroach and their antiangiogenic activity. Org. Chem. Front. 2021, 8, 1401–1408. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 24 September 2022).
- Sheikh, O.; Yokota, T. Restoring Protein Expression in Neuromuscular Conditions: A Review Assessing the Current State of Exon Skipping/Inclusion and Gene Therapies for Duchenne Muscular Dystrophy and Spinal Muscular Atrophy. BioDrugs 2021, 35, 389–399. [Google Scholar] [CrossRef]
- Lenci, E.; Calugi, L.; Trabocchi, A. Occurrence of Morpholine in Central Nervous System Drug Discovery. ACS Chem. Neurosci. 2021, 12, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Tzara, A.; Xanthopoulos, D.; Kourounakis, A.P. Morpholine As a Scaffold in Medicinal Chemistry: An Update on Synthetic Strategies. Chem. Med. Chem. 2020, 15, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Kourounakis, A.P.; Xanthopoulos, D.; Tzara, A. Morpholine as a privileged structure: A review on the medicinal chemistry and pharmacological activity of morpholine containing bioactive molecules. Med. Res. Rev. 2020, 40, 709–752. [Google Scholar] [CrossRef]
- Pourshojaei, Y.; Abiri, A.; Eskandari, K.; Haghighijoo, Z.; Edraki, N.; Asadipour, A. Phenoxyethyl Piperidine/Morpholine Derivatives as PAS and CAS Inhibitors of Cholinesterases: Insights for Future Drug Design. Sci. Rep. 2019, 9, 19855. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, R.; Eom, B.H.; Heo, J.H.; Park, J.E.; Abdelgawad, M.A.; Musa, A.; Gambacorta, N.; Nicolotti, O.; Manju, S.L.; Mathew, B.; et al. Morpholine-based chalcones as dual-acting monoamine oxidase-B and acetylcholinesterase inhibitors: Synthesis and biochemical investigations. J. Enzyme. Inhib. Med. Chem. 2021, 36, 188–197. [Google Scholar] [CrossRef]
- Prikhodko, V.A.; Sysoev, Y.I.; Okovityi, S.V. Morpholine derivatives as potential agents for neurological manifestations of nervous system diseases. Pharm. Formulas 2020, 2, 16–35. [Google Scholar] [CrossRef]
- Chernov, N.M.; Shutov, R.V.; Barygin, O.I.; Dron, M.Y.; Starova, G.L.; Kuz’mich, N.N.; Yakovlev, I.P. Synthesis of Chromone-Containing Allylmorpholines through a Morita–Baylis–Hillman-Type Reaction. Eur. J. Org. Chem. 2018, 45, 6304–6313. [Google Scholar] [CrossRef]
- Li, V.; Wang, Y.T. Molecular mechanisms of NMDA receptor-mediated excitotoxicity: Implications for neuroprotective therapeutics for stroke. Neural. Regen. Res. 2016, 11, 1752–1753. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.O.; Lee, S.J.; Pyo, J.S. Effect of acetylcholinesterase inhibitors on post-stroke cognitive impairment and vascular dementia: A meta-analysis. PLoS ONE 2020, 15, e0227820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remya, C.; Dileep, K.V.; Koti Reddy, E.; Mantosh, K.; Lakshmi, K.; Sarah Jacob, R.; Sajith, A.M.; Jayadevi Variyar, E.; Anwar, S.; Zhang, K.; et al. Neuroprotective derivatives of tacrine that target NMDA receptor and acetyl cholinesterase—Design, synthesis and biological evaluation. Comput. Struct. Biotechnol. J. 2021, 19, 4517–4537. [Google Scholar] [CrossRef]
- Prikhodko, V.A.; Kan, A.V.; Sysoev, Y.I.; Titovich, I.A.; Anisimova, N.A.; Okovityi, S.V. Evaluation of the Neuroprotective Activity of a New Allylmorpholine Derivative in a Rat Model of Traumatic Brain Injury. Drug Dev. Regist. 2021, 10, 179–187. [Google Scholar] [CrossRef]
- Bertrand, C.; Chatonnet, A.; Takke, C.; Yan, Y.L.; Postlethwait, J.; Toutant, J.P.; Cousin, X. Zebrafish acetylcholinesterase is encoded by a single gene localized on linkage group 7. Gene structure and polymorphism; molecular forms and expression pattern during development. J. Biol. Chem. 2001, 276, 464–474. [Google Scholar] [CrossRef] [Green Version]
- Horzmann, K.A.; Freeman, J.L. Zebrafish Get Connected: Investigating Neurotransmission Targets and Alterations in Chemical Toxicity. Toxics 2016, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Santana, S.; Rico, E.P.; Burgos, J.S. Can zebrafish be used as animal model to study Alzheimer’s disease? Am. J. Neurodegener. Dis. 2012, 1, 32–48. [Google Scholar]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain. Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and husbandry recommendations. Lab. Anim. 2020, 54, 213–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, G.E. Zebrafish housing, husbandry, health, and care: IACUC considerations. ILAR J. 2012, 53, 205–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, F.V.; Canzian, J.; Stefanello, F.V.; Kalueff, A.V.; Rosemberg, D.B. Naloxone prolongs abdominal constriction writhing-like behavior in a zebrafish-based pain model. Neurosci. Lett. 2019, 708, 134336. [Google Scholar] [CrossRef] [PubMed]
- Angiulli, E.; Pagliara, V.; Cioni, C.; Frabetti, F.; Pizzetti, F.; Alleva, E.; Toni, M. Increase in environmental temperature affects exploratory behaviour, anxiety and social preference in Danio rerio. Sci. Rep. 2020, 10, 5385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghani, S.; Karia, M.; Cheng, R.K.; Mathuru, A.S. An Automated Assay System to Study Novel Tank Induced Anxiety. Front. Behav. Neurosci. 2019, 13, 180. [Google Scholar] [CrossRef] [Green Version]
- Fontana, B.D.; Alnassar, N.; Parker, M.O. The zebrafish (Danio rerio) anxiety test battery: Comparison of behavioral responses in the novel tank diving and light-dark tasks following exposure to anxiogenic and anxiolytic compounds. Psychopharmacology 2022, 239, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Sinyakova, N.A.; Kulikova, E.A.; Englevskii, N.A.; Kulikov, A.V. Effects of Fluoxetine and Potential Antidepressant 8-Trifluoromethyl 1,2,3,4,5-Benzopentathiepin-6-Amine Hydrochloride (TC-2153) on Behavior of Danio rerio Fish in the Novel Tank Test and Brain Content of Biogenic Amines and Their Metabolites. Bull. Exp. Biol. Med. 2018, 164, 620–623. [Google Scholar] [CrossRef]
- Blaser, R.E.; Rosemberg, D.B. Measures of anxiety in zebrafish (Danio rerio): Dissociation of black/white preference and novel tank test. PLoS ONE 2012, 7, e36931. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Ugalde Muñiz, P.; Pineda, B.; Pedraza-Chaverrí, J.; Ríos, C.; Pérez-de la Cruz, V. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxid. Med. Cell Longev. 2013, 2013, 104024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St’astný, F.; Lisý, V.; Mares, V.; Lisá, V.; Balcar, V.J.; Santamaría, A. Quinolinic acid induces NMDA receptor-mediated lipid peroxidation in rat brain microvessels. Redox. Rep. 2004, 9, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Maximino, C.; de Brito, T.M.; Herculano, A.M.; Gouveia, A., Jr.; Morato, S.; Cachat, J.M.; Gaikwad, S.; Elegante, M.F.; Hart, P.C.; et al. Neurophenotyping of Adult Zebrafish Using the Light/Dark Box Paradigm. In Zebrafish Neurobehavioral Protocols; Kalueff, A.V., Cachat, J.M., Eds.; Springer: Totowa, NJ, USA, 2010; pp. 157–167. [Google Scholar] [CrossRef]
- Mansur, B.; Dos Santos, B.R.; Dias, C.A.; Pinheiro, M.; Gouveia, A., Jr. Effects of the number of subjects on the dark/light preference of Zebrafish (Danio rerio). Zebrafish 2014, 11, 560–566. [Google Scholar] [CrossRef]
- Champagne, D.L.; Hoefnagels, C.C.; de Kloet, R.E.; Richardson, M.K. Translating rodent behavioral repertoire to zebrafish (Danio rerio): Relevance for stress research. Behav. Brain Res. 2010, 214, 332–342. [Google Scholar] [CrossRef] [PubMed]
- BLAST: Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 20 October 2022).
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Gene. Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 20 October 2022).
- RCSB Protein Data Bank. Available online: https://www.rcsb.org/ (accessed on 20 October 2022).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genario, R.; Giacomini, A.C.V.V.; de Abreu, M.S.; Marcon, L.; Demin, K.A.; Kalueff, A.V. Sex differences in adult zebrafish anxiolytic-like responses to diazepam and melatonin. Neurosci. Lett. 2020, 714, 134548. [Google Scholar] [CrossRef]
- Bencan, Z.; Sledge, D.; Levin, E.D. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol. Biochem. Behav. 2009, 94, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Maximino, C.; da Silva, A.W.; Araújo, J.; Lima, M.G.; Miranda, V.; Puty, B.; Benzecry, R.; Picanço-Diniz, D.L.; Gouveia, A., Jr.; Oliveira, K.R.; et al. Fingerprinting of psychoactive drugs in zebrafish anxiety-like behaviors. PLoS ONE 2014, 9, e103943. [Google Scholar] [CrossRef]
- Vossen, L.E.; Brunberg, R.; Rådén, P.; Winberg, S.; Roman, E. Sex-Specific Effects of Acute Ethanol Exposure on Locomotory Activity and Exploratory Behavior in Adult Zebrafish (Danio rerio). Front. Pharmacol. 2022, 13, 853936. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Broeyer, F.; de Kam, M.; Baas, J.; Cohen, A.; van Gerven, J. Pharmacodynamic response profiles of anxiolytic and sedative drugs. Br. J. Clin. Pharmacol. 2017, 83, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.S. Opioid metabolism. Mayo. Clin. Proc. 2009, 84, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Bertilsson, L. Clinical pharmacokinetics of carbamazepine. Clin. Pharmacokinet. 1978, 3, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, R.E.; Habersang, R.; Lansky, L. Kinetics of primidone metabolism and excretion in children. Clin. Pharmacol. Ther. 1977, 22, 200–205. [Google Scholar] [CrossRef]
- Michelotti, P.; Quadros, V.A.; Pereira, M.E.; Rosemberg, D.B. Ketamine modulates aggressive behavior in adult zebrafish. Neurosci. Lett. 2018, 684, 164–168. [Google Scholar] [CrossRef]
- Kolesnikova, T.O.; Khatsko, S.L.; Shevyrin, V.A.; Morzherin, Y.Y.; Kalueff, A.V. Effects of a non-competitive N-methyl-d-aspartate (NMDA) antagonist, tiletamine, in adult zebrafish. Neurotoxicol. Teratol. 2017, 59, 62–67. [Google Scholar] [CrossRef]
- Menezes, F.P.; Kist, L.W.; Bogo, M.R.; Bonan, C.D.; Da Silva, R.S. Evaluation of age-dependent response to NMDA receptor antagonism in zebrafish. Zebrafish 2015, 12, 137–143. [Google Scholar] [CrossRef]
- Woods, I.G.; Wilson, C.; Friedlander, B.; Chang, P.; Reyes, D.K.; Nix, R.; Kelly, P.D.; Chu, F.; Postlethwait, J.H.; Talbot, W.S. The zebrafish gene map defines ancestral vertebrate chromosomes. Genome Res. 2005, 15, 1307–1314. [Google Scholar] [CrossRef] [Green Version]
- Rico, E.P.; Rosemberg, D.B.; Seibt, K.J.; Capiotti, K.M.; Da Silva, R.S.; Bonan, C.D. Zebrafish neurotransmitter systems as potential pharmacological and toxicological targets. Neurotoxicol. Teratol. 2011, 33, 608–617. [Google Scholar] [CrossRef]
- Giacomini, A.C.; Bueno, B.W.; Marcon, L.; Scolari, N.; Genario, R.; Demin, K.A.; Kolesnikova, T.O.; Kalueff, A.V.; de Abreu, M.S. An acetylcholinesterase inhibitor, donepezil, increases anxiety and cortisol levels in adult zebrafish. J. Psychopharmacol. 2020, 34, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Lee, C.-J.; Choi, J.; Hwang, J.; Lee, Y. Anxiolytic effects of an acetylcholinesterase inhibitor, physostigmine, in the adult zebrafish. Anim. Cells Syst. 2012, 16, 198–206. [Google Scholar] [CrossRef]
- Karunakaran, K.B.; Thiyagaraj, A.; Santhakumar, K. Novel insights on acetylcholinesterase inhibition by Convolvulus pluricaulis, scopolamine and their combination in zebrafish. Nat. Prod. Bioprospect. 2022, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Rima, M.; Lattouf, Y.; Abi Younes, M.; Bullier, E.; Legendre, P.; Mangin, J.M.; Hong, E. Dynamic regulation of the cholinergic system in the spinal central nervous system. Sci. Rep. 2020, 10, 15338. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.Y.; Mathur, P.; Gould, G.G.; Guo, S. Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc. Natl. Acad. Sci. USA 2011, 108, 2581–2586. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Expósito, B.; Gómez, A.; Martín-Monzón, I.; Reiriz, M.; Rodríguez, F.; Salas, C. Goldfish hippocampal pallium is essential to associate temporally discontiguous events. Neurobiol. Learn. Mem. 2017, 139, 128–134. [Google Scholar] [CrossRef]
- Mans, R.A.; Hinton, K.D.; Payne, C.H.; Powers, G.E.; Scheuermann, N.L.; Saint-Jean, M. Cholinergic Stimulation of the Adult Zebrafish Brain Induces Phosphorylation of Glycogen Synthase Kinase-3 β and Extracellular Signal-Regulated Kinase in the Telencephalon. Front. Mol. Neurosci. 2019, 12, 91. [Google Scholar] [CrossRef]
- Koenig, J.A.; Dao, T.L.; Kan, R.K.; Shih, T.M. Zebrafish as a model for acetylcholinesterase-inhibiting organophosphorus agent exposure and oxime reactivation. Ann. N. Y. Acad. Sci. 2016, 1374, 68–77. [Google Scholar] [CrossRef]














| Original Name | Name | Structure | IC50, μM; or % Inhibition at 24 μM | ||
|---|---|---|---|---|---|
| eqBChE | eeAChE | NMDAR | |||
| 9a | 1 |  | 14.50 ± 0.40 | 15% | 28.00 ± 6.00 |
| 9j | 2 |  | 1.20 ± 0.10 | 7.00 ± 1.00 | 51.00 ± 12.00 |
| 9l | 3 |  | 17% | 6.40 ± 0.20 | nd |
| 33a | 4 |  | 1.60 ± 0.10 | 14% | 15.00 ± 4.00 |
| 33b | 5 |  | 0.42 ± 0.03 | 7.00 ± 1.00 | 8.00 ± 3.00 |
| Experiment | CCAM | Putative Antagonist | Concentrations, mg·L−1 | |||||
|---|---|---|---|---|---|---|---|---|
| CCAM | Putative Antagonist | |||||||
| a | b | c | a | b | c | |||
| 1 | 1 | - | 1 | 10 | 20 a | - | ||
| 2 | 2 | - | 1 | 10 | 100 | - | ||
| 3 | 3 | - | 1 | 10 | 100 | - | ||
| 4 | 4 | - | 1 | 10 | 100 | - | ||
| 5 | 5 | - | 0.001 a | 0.01 a | 0.1 a | - | ||
| 6 | 1 | NMDA | 20 | 3.83 b | ||||
| 7 | - | NMDA | - | 100 | ||||
| 8 | - | quinolinic acid | - | 10 | 50 | 100 | ||
| 9 | 1 | biperiden | 20 | 8.11 b | ||||
| 10, 11 | 4 | - | 0.5 | 1 | 5 | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prikhodko, V.A.; Sysoev, Y.I.; Gerasimova, E.V.; Okovityi, S.V. Novel Chromone-Containing Allylmorpholines Induce Anxiolytic-like and Sedative Effects in Adult Zebrafish. Biomedicines 2022, 10, 2783. https://doi.org/10.3390/biomedicines10112783
Prikhodko VA, Sysoev YI, Gerasimova EV, Okovityi SV. Novel Chromone-Containing Allylmorpholines Induce Anxiolytic-like and Sedative Effects in Adult Zebrafish. Biomedicines. 2022; 10(11):2783. https://doi.org/10.3390/biomedicines10112783
Chicago/Turabian StylePrikhodko, Veronika A., Yuri I. Sysoev, Elena V. Gerasimova, and Sergey V. Okovityi. 2022. "Novel Chromone-Containing Allylmorpholines Induce Anxiolytic-like and Sedative Effects in Adult Zebrafish" Biomedicines 10, no. 11: 2783. https://doi.org/10.3390/biomedicines10112783