Metformin Prevents Endothelial Dysfunction in Endometriosis through Downregulation of ET-1 and Upregulation of eNOS
Abstract
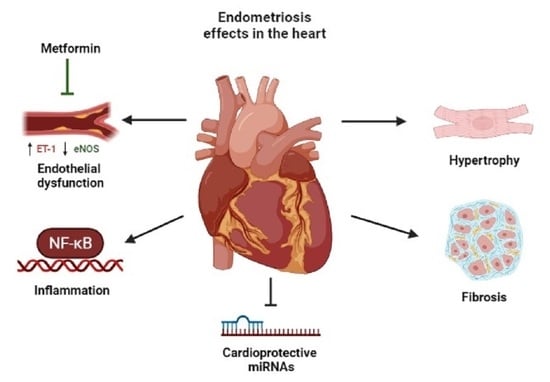
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Computer-Assisted Histologic Study of the Heart
2.3. Dual Immunolabelling in Heart Sections
2.4. Western Blotting
2.5. Real-Time qPCR
2.6. Statistical Analysis
3. Results
3.1. Endometriosis Increases Cardiac Fibrosis and Metformin Increases Cardiomyocyte Cross-Sectional Area
3.2. ET-1 and VEGF Are Expressed in the Vascular Smooth Muscle Cells and VEGFR-2, eNOS and iNOS in the Endothelium
3.3. Endometriosis Increases NF-kB Expression in the Heart and Metformin Attenuates Endothelial Dysfunction through ET-1 Downregulation and eNOS Upregulation
3.4. Endometriosis Downregulates Expression of Cardioprotective MIR199a, MIR16-1 and MIR18a
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Mu, F.; Rich-Edwards, J.; Rimm, E.B.; Spiegelman, D.; Forman, J.P.; Missmer, S.A. Association Between Endometriosis and Hypercholesterolemia or Hypertension. Hypertension 2017, 70, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Mu, F.; Rich-Edwards, J.; Rimm, E.B.; Spiegelman, D.; Missmer, S.A. Endometriosis and Risk of Coronary Heart Disease. Circul. Cardiovasc. Qual. Outcomes 2016, 9, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafi, U.; Ahmad, S.; Bokhari, S.S.; Iqbal, M.A.; Zia, A.; Khan, M.A.; Roohi, N. Association of Inflammatory Markers/Cytokines with Cardiovascular Risk Manifestation in Patients with Endometriosis. Mediat. Inflamm. 2021, 2021, 3425560. [Google Scholar] [CrossRef]
- Kvaskoff, M.; Mu, F.; Terry, K.L.; Harris, H.R.; Poole, E.M.; Farland, L.; Missmer, S.A. Endometriosis: A high-risk population for major chronic diseases? Hum. Reprod. Update 2015, 21, 500–516. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Taskin, O.; Iews, M.; Lee, A.J.; Kan, A.; Rowe, T.; Bedaiwy, M.A. Atherosclerotic cardiovascular disease in women with endometriosis: A systematic review of risk factors and prospects for early surveillance. Reprod. Biomed. Online 2019, 39, 1007–1016. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Ding, D.; Liu, X.; Guo, S.-W. Evidence for a hypercoagulable state in women with ovarian endometriomas. Reprod. Sci. 2015, 22, 1107–1114. [Google Scholar] [CrossRef]
- Maeda, E.; Koshiba, A.; Mori, T.; Ito, F.; Kataoka, H.; Okimura, H.; Sugahara, T.; Tarumi, Y.; Kusuki, I.; Khan, K.N. Atherosclerosis-related biomarkers in women with endometriosis: The effects of dienogest and oral contraceptive therapy. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2020, 7, 100108. [Google Scholar] [CrossRef]
- Bohm, F.; Pernow, J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 2007, 76, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, O.; Yamada-Nomoto, K.; Kobayashi, M.; Andoh, T.; Hongo, M.; Ono, Y.; Hasegawa-Idemitsu, A.; Sakai, A.; Osuga, Y.; Saito, S. Bradykinin system is involved in endometriosis-related pain through endothelin-1 production. Eur. J. Pain 2018, 22, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, S.; Shinohara, K.; Wakatsuki, A. Increased asymmetric dimethylarginine and enhanced inflammation are associated with impaired vascular reactivity in women with endometriosis. Atherosclerosis 2011, 219, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Paneni, F.; Cosentino, F. Ageing, metabolism and cardiovascular disease. J. Physiol. 2016, 594, 2061–2073. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Wu, M.-H.; Hsiao, K.-Y.; Tsai, S.-J. Endometriosis and possible inflammation markers. Gynecol. Minim. Invasive Ther. 2015, 4, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.L.L.; Reis, F.M.; Taylor, R.N. Angiogenesis and Endometriosis. Obstet. Gynecol. Int. 2013, 2013, 859619. [Google Scholar] [CrossRef] [Green Version]
- Khurana, R.; Simons, M.; Martin, J.F.; Zachary, I.C. Role of Angiogenesis in Cardiovascular Disease. Circulation 2005, 112, 1813–1824. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Moustafa, S.; Burn, M.; Mamillapalli, R.; Nematian, S.; Flores, V.; Taylor, H.S. Accurate diagnosis of endometriosis using serum microRNAs. Am. J. Obstet. Gynecol. 2020, 223, 557.e1–557.e11. [Google Scholar] [CrossRef]
- Cosar, E.; Mamillapalli, R.; Ersoy, G.S.; Cho, S.; Seifer, B.; Taylor, H.S. Serum microRNAs as diagnostic markers of endometriosis: A comprehensive array-based analysis. Fertil. Steril. 2016, 106, 402–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Li, H.; Yuan, M.; Li, D.; Sun, C.; Wang, G. Serum Exosomal MicroRNAs as Potential Circulating Biomarkers for Endometriosis. Dis. Markers 2020, 2020, 2456340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maged, A.M.; Deeb, W.S.; El Amir, A.; Zaki, S.S.; El Sawah, H.; Al Mohamady, M.; Metwally, A.A.; Katta, M.A. Diagnostic accuracy of serum miR-122 and miR-199a in women with endometriosis. Int. J. Gynecol. Obstet. 2018, 141, 14–19. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Y.; Guan, L.; Elkin, K.; Xiao, J. Targets identified from exercised heart: Killing multiple birds with one stone. npj Regen. Med. 2021, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Boon, R.A.; Dimmeler, S. MicroRNAs in myocardial infarction. Nat. Rev. Cardiol. 2015, 12, 135–142. [Google Scholar] [CrossRef]
- Ren, X.-P.; Wu, J.; Wang, X.; Sartor, M.A.; Qian, J.; Jones, K.; Nicolaou, P.; Pritchard, T.J.; Fan, G.-C. MicroRNA-320 Is Involved in the Regulation of Cardiac Ischemia/Reperfusion Injury by Targeting Heat-Shock Protein 20. Circulation 2009, 119, 2357–2366. [Google Scholar] [CrossRef] [Green Version]
- Kir, D.; Schnettler, E.; Modi, S.; Ramakrishnan, S. Regulation of angiogenesis by microRNAs in cardiovascular diseases. Angiogenesis 2018, 21, 699–710. [Google Scholar] [CrossRef]
- Liu, X.; Guo, B.; Zhang, W.; Ma, B.; Li, Y. MiR-20a-5p overexpression prevented diabetic cardiomyopathy via inhibition of cardiomyocyte apoptosis, hypertrophy, fibrosis and JNK/NF-κB signalling pathway. J. Biochem. 2021, 170, 349–362. [Google Scholar] [CrossRef]
- Sun, X.; Zuo, H.; Liu, C.; Yang, Y. Overexpression of miR-200a protects cardiomyocytes against hypoxia-induced apoptosis by modulating the kelch-like ECH-associated protein 1-nuclear factor erythroid 2-related factor 2 signaling axis. Int. J. Mol. Med. 2016, 38, 1303–1311. [Google Scholar] [CrossRef] [Green Version]
- Ferrero, S.; Evangelisti, G.; Barra, F. Current and emerging treatment options for endometriosis. Expert Opin. Pharmacother. 2018, 19, 1109–1125. [Google Scholar] [CrossRef]
- Kalaitzopoulos, D.R.; Samartzis, N.; Kolovos, G.N.; Mareti, E.; Samartzis, E.P.; Eberhard, M.; Dinas, K.; Daniilidis, A. Treatment of endometriosis: A review with comparison of 8 guidelines. BMC Women Health 2021, 21, 397. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Guan, K.-L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers-Broadway, K.R.; Kumar, J.; Sisu, C.; Wander, G.; Mazey, E.; Jeyaneethi, J.; Pados, G.; Tsolakidis, D.; Klonos, E.; Grunt, T.; et al. Differential expression of mTOR components in endometriosis and ovarian cancer: Effects of rapalogues and dual kinase inhibitors on mTORC1 and mTORC2 stoichiometry. Int. J. Mol. Med. 2018, 43, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kimber-Trojnar, Ż.; Dłuski, D.F.; Wierzchowska-Opoka, M.; Ruszała, M.; Leszczyńska-Gorzelak, B. Metformin as a potential treatment option for endometriosis. Cancers 2022, 14, 577. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, B.; Sucak, A.; Kilic, S.; Aksakal, O.; Aksoy, Y.; Lortlar, N.; Sut, N.; Gungor, T. Metformin regresses endometriotic implants in rats by improving implant levels of superoxide dismutase, vascular endothelial growth factor, tissue inhibitor of metalloproteinase-2, and matrix metalloproteinase-9. Am. J. Obstet. Gynecol. 2010, 202, 368.e1–368.e8. [Google Scholar] [CrossRef]
- Cameron, A.R.; Morrison, V.L.; Levin, D.; Mohan, M.; Forteath, C.; Beall, C.; McNeilly, A.D.; Balfour, D.J.K.; Savinko, T.; Wong, A.K.F.; et al. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ. Res. 2016, 119, 652–665. [Google Scholar] [CrossRef] [Green Version]
- Martin-Montalvo, A.; Mercken, E.M.; Mitchell, S.J.; Palacios, H.H.; Mote, P.L.; Scheibye-Knudsen, M.; Gomes, A.P.; Ward, T.M.; Minor, R.K.; Blouin, M.-J.; et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013, 4, 2192. [Google Scholar] [CrossRef] [Green Version]
- Mansfield, R.; Galea, R.; Brincat, M.; Hole, D.; Mason, H. Metformin has direct effects on human ovarian steroidogenesis. Fertil. Steril. 2003, 79, 956–962. [Google Scholar] [CrossRef]
- Yari, S.; Khoei, H.H.; Saber, M.; Esfandiari, F.; Moini, A.; Shahhoseini, M. Metformin attenuates expression of angiogenic and inflammatory genes in human endometriotic stromal cells. Exp. Cell Res. 2021, 404, 112659. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, L.; Long, Y.; Pei, T.; Luo, B.; Liao, T.; Li, Y.; Zhu, H.; Ouyang, Y.; Huang, W. Comparative Proteomic Analysis Reveals Metformin Improves the Expression of Biomarkers of Endometrial Receptivity in Infertile Women with Minimal/Mild Endometriosis. Reprod. Sci. 2022, 29, 2593–2606. [Google Scholar] [CrossRef] [PubMed]
- Foda, A.A.; Aal, I.A.A. Metformin as a new therapy for endometriosis, its effects on both clinical picture and cytokines profile. Middle East Fertil. Soc. J. 2012, 17, 262–267. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.; Ziyyat, A.; Naoura, I.; Chabbert-Buffet, N.; Aractingi, S.; Darai, E.; Lefevre, B. Effect of induced peritoneal endometriosis on oocyte and embryo quality in a mouse model. J. Assist. Reprod. Genet. 2015, 32, 263–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, L.; D’Onofrio, F.; Campo, S.; Ferraro, P.M.; Tondi, P.; Campo, V.; Flex, A.; Gasbarrini, A.; Santoliquido, A. Endothelial dysfunction but not increased carotid intima-media thickness in young European women with endometriosis. Hum. Reprod. 2012, 27, 1320–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frangogiannis, N.G. Cardiac fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef]
- Bai, B.; Chen, H. Metformin: A novel weapon against inflammation. Front. Pharmacol. 2021, 12, 622262. [Google Scholar] [CrossRef]
- Yenmis, G.; Sarac, E.Y.; Besli, N.; Soydas, T.; Tastan, C.; Kancagi, D.D.; Yilanci, M.; Senol, K.; Karagulle, O.O.; Ekmekci, C.G. Anti-cancer effect of metformin on the metastasis and invasion of primary breast cancer cells through mediating NF-kB activity. Acta Histochem. 2021, 123, 151709. [Google Scholar] [CrossRef]
- Kessler, E.L.; Rivaud, M.R.; Vos, M.A.; van Veen, T.A. Sex-specific influence on cardiac structural remodeling and therapy in cardiovascular disease. Biol. Sex Differ. 2019, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Fu, Y.-N.; Xiao, H.; Ma, X.-W.; Jiang, S.-Y.; Xu, M.; Zhang, Y.-Y. Metformin attenuates pressure overload-induced cardiac hypertrophy via AMPK activation. Acta Pharmacol. Sin. 2011, 32, 879–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, M.; Al-Talabany, S.; McKinnie, A.; Mordi, I.R.; Singh, J.S.S.; Gandy, S.J.; Baig, F.; Hussain, M.S.; Bhalraam, U.; Khan, F.; et al. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: The MET-REMODEL trial. Eur. Heart J. 2019, 40, 3409–3417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, J.S.; Barreto-Torres, G.; Kuznetsov, A.V.; Khuchua, Z.; Javadov, S. Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: The role of mitochondria. J. Cell. Mol. Med. 2014, 18, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, Y.; Zhao, H.; Wang, Q.; Chen, P. The histone demethylase JMJD1C regulates CAMKK2-AMPK signaling to participate in cardiac hypertrophy. Front. Physiol. 2020, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shen, W.; Liu, Z.; Sheng, S.; Xiong, W.; He, R.; Zhang, X.; Ma, L.; Ju, Z. Effect of metformin on cardiac metabolism and longevity in aged female mice. Front. Cell Dev. Biol. 2021, 8, 626011. [Google Scholar] [CrossRef] [PubMed]
- Mahnke, J.L.; Dawood, M.Y.; Huang, J.-C. Vascular endothelial growth factor and interleukin-6 in peritoneal fluid of women with endometriosis. Fertil. Steril. 2000, 73, 166–170. [Google Scholar] [CrossRef]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 2019, 10, 637. [Google Scholar] [CrossRef] [Green Version]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Wehbe, N.; Nasser, S.; Pintus, G.; Badran, A.; Eid, A.; Baydoun, E. MicroRNAs in Cardiac Hypertrophy. Int. J. Mol. Sci. 2019, 20, 4714. [Google Scholar] [CrossRef]




| Primary Antibodies | Concentration (v/v) |
|---|---|
| ET-1—Goat IgG Polyclonal-Santa Cruz Biotech, Dallas, Texas, USA. ref. sc-21625 | 1:100 |
| VEGF—Goat IgG Polyclonal-R&D, Minneapolis, Minnesota, USA. ref. AF564 | 1:50 |
| VEGFR-2—Rabbit IgG Polyclonal-Santa Cruz Biotech. ref. sc-504 | 1:100 |
| iNOS—Rabbit IgG Polyclonal—Abcam Cambridge, UK. ref. ab178945 | 1:200 |
| eNOS—Rabbit IgG Monoclonal-Santa Cruz Biotech. ref. sc-7271 | 1:200 |
| α-SMA—Mouse IgG Monoclonal—Milllipore, Burlington, Massachusetts, USA. ref. CBL171 | 1:300 |
| Secondary Antibodies | Concentration (v/v) |
| Anti-Goat Alexa Fluor 568 Molecular Probes Engene, Oregon, USA. ref. A11057 | 1:2000 |
| Anti-Rabbit Alexa Fluor 488 Molecular Probes ref. A21206 | 1:2000 |
| Anti-Mouse Alexa Fluor 568 Molecular Probes ref. A10037 | 1:2000 |
| Primary Antibodies | Concentration (v/v) | % Acrylamide Resolving Gel |
|---|---|---|
| ET-1—Rabbit IgG Polyclonal-R&D ref. sc-21625-R | 1:1000 | 12 |
| eNOS—Rabbit IgG Monoclonal-Cell Signaling Technology, Danvers, Massachusetts, USA. ref. D9A5L | 1:1000 | 10 |
| VEGF—Mouse IgG Monoclonal R&D ref. MAB564 | 1:100 | 14 |
| NF-kB—Rabbit IgG Monoclonal-Cell Signaling Tech. ref. D14E12 | 1:1000 | 10 |
| Ikβα—Mouse IgG Monoclonal-Cell Signaling Tech. ref. L35A5 | 1:1000 | 10 |
| keap-1—Rabbit IgG Monoclonal-Cell Signaling Tech. ref. D6B12 | 1:1000 | 12 |
| Secondary Antibodies | Concentration (v/v) | |
| Goat Anti-Mouse IgG-HRP-Santa Cruz Biotech. ref. sc-2005 | 1:10,000 | |
| Donkey Anti-Rabbit IgG-HRP-Jackson ImmunoResearch, West Grove, Pennsylvania, USA. ref. 711-035-152 | 1:5000 | |
| MiRNA | Reference |
|---|---|
| RNU | Hs_RNU6-2_1 miScript Primer Assay (ref. MS00033740) |
| MIR199a | Hs_miR-199a_1 miScript Primer Assay (ref. MS00006741) |
| MIR16-1 | has_miR-16-5p miRCURY LNA™ miRNA PCR Assay (ref. YP00205702) |
| MIR18a | has_miR-18a-5p miRCURY LNA™ miRNA PCR Assay (ref. YP00204207) |
| MIR20a | Hs_miR-20a_1 miScript Primer Assay (ref. MS00003199) |
| MIR155 | Mm_miR-155_1 miScript Primer Assay (ref. MS00001701) |
| MIR342 | has_miR-342-3p miRCURY LNA™ miRNA PCR Assay (ref. YP00205625) |
| MIR200a | Hs_miR-200a_1 miScript Primer Assay (ref. MS00003738) |
| MIR24-1 | has_miR-24-3p miRCURY LNA™ miRNA PCR Assay (ref. YP00204260) |
| MIR320a | Hs_miR-320a_1 miScript Primer Assay (ref. MS00014707) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, A.F.; Neto, A.C.; Rodrigues, A.R.; Oliveira, S.M.; Sousa-Mendes, C.; Leite-Moreira, A.; Gouveia, A.M.; Almeida, H.; Neves, D. Metformin Prevents Endothelial Dysfunction in Endometriosis through Downregulation of ET-1 and Upregulation of eNOS. Biomedicines 2022, 10, 2782. https://doi.org/10.3390/biomedicines10112782
Martins AF, Neto AC, Rodrigues AR, Oliveira SM, Sousa-Mendes C, Leite-Moreira A, Gouveia AM, Almeida H, Neves D. Metformin Prevents Endothelial Dysfunction in Endometriosis through Downregulation of ET-1 and Upregulation of eNOS. Biomedicines. 2022; 10(11):2782. https://doi.org/10.3390/biomedicines10112782
Chicago/Turabian StyleMartins, Ana Filipa, Ana Catarina Neto, Adriana Raquel Rodrigues, Sandra Marisa Oliveira, Cláudia Sousa-Mendes, Adelino Leite-Moreira, Alexandra Maria Gouveia, Henrique Almeida, and Delminda Neves. 2022. "Metformin Prevents Endothelial Dysfunction in Endometriosis through Downregulation of ET-1 and Upregulation of eNOS" Biomedicines 10, no. 11: 2782. https://doi.org/10.3390/biomedicines10112782