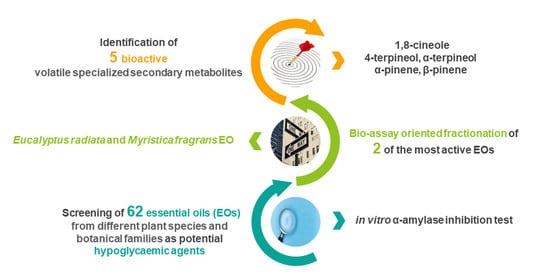
Bio-Guided Fractionation Driven by In Vitro α-Amylase Inhibition Assays of Essential Oils Bearing Specialized Metabolites with Potential Hypoglycemic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the In Vitro Enzymatic Test
2.2. In Vitro α-Amylase Inhibition Test
2.3. Essential Oil Composition
2.4. Bio-Guided Assay Fractionation of Eucalyptus radiata and Myristica fragrans
2.5. Data Precision
3. Materials and Methods
3.1. Reagents
3.2. In Vitro α-Amylase Inhibition Test
3.3. Maltose Calibration Curve
3.4. Flash Column Chromatography
3.5. Analysis Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, C.J. New Approaches to the pharmacotherapy of diabetes. In Textbook of Diabetes; Pickup, J., William, G., Eds.; Blackwell Science Ltd.: Oxford, UK, 2003; pp. 73.1–73.21. [Google Scholar]
- Fujisawa, T.; Ikegami, H.; Inoue, K.; Kawabata, Y.; Ogihara, T. Effect of two α-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism 2005, 54, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.; Francini, F.; Schinella, G. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedekar, A.; Shah, K.; Koffas, M. Natural products for type II diabetes treatment. Adv. Appl. Microbiol. 2010, 71, 21–73. [Google Scholar] [PubMed]
- Nelson-Dooley, C.; Della-Fera, M.; Hamrick, M.; Baile, C. Novel Treatments for Obesity and Osteoporosis: Targeting Apoptotic Pathways in Adipocytes. Curr. Med. Chem. 2005, 12, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Ueda, T.; Oki, T.; Sugita, K.; Terahara, N.; Matsumoto, K. α-glucosidase inhibitory action of natural acylated anthocyanins. 1. Survey of natural pigments with potent inhibitory activity. J. Agric. Food Chem. 2001, 49, 1948–1951. [Google Scholar] [CrossRef] [PubMed]
- Can Başer, K.H.; Buchbauer, G. Handbook of Essential Oils; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Goerg, K.J.; Spilker, T. Effect of peppermint oil and caraway oil on gastrointestinal motility in healthy volunteers: A pharmacodynamic study using simultaneous determination of gastric and gall-bladder emptying and orocaecal transit time. Aliment Pharmacol. Ther. 2003, 17, 445–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappello, G.; Spezzaferro, M.; Grossi, L.; Manzoli, L.; Marzio, L. Peppermint oil (Mintoil®) in the treatment of irritable bowel syndrome: A prospective double blind placebo-controlled randomized trial. Dig. Liver Dis. 2007, 39, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Kenia, P.; Houghton, T.; Beardsmore, C. Does inhaling menthol affect nasal patency or cough? Pediatr. Pulmonol. 2008, 43, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Kehrl, W.; Sonnemann, U.; Dethlefsen, U. Therapy for Acute Nonpurulent Rhinosinusitis with Cineole: Results of a Double-Blind, Randomized, Placebo-Controlled Trial. Laryngoscope 2004, 114, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, J.S. Method for Treating Gastrointestinal Disorders. US Patent USOO6420435B1, 16 July 2002. [Google Scholar]
- Kim, D.H.; Goh, H.J.; Lee, H.W.; Kim, K.S.; Kim, Y.T.; Moon, H.S.; Lee, S.W.; Park, S.Y. The effect of terpene combination on ureter calculus expulsion after extracorporeal shock wave lithotripsy. Korean J. Urol. 2014, 55, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Romics, I.; Siller, G.; Kohnen, R.; Mavrogenis, S.; Varga, J.; Holman, E. A Special Terpene Combination (Rowatinex®) Improves Stone Clearance after Extracorporeal Shockwave Lithotripsy in Urolithiasis Patients: Results of a Placebo-Controlled Randomised Controlled Trial. Urol. Int. 2011, 86, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K. Antidiabetic potential of plant natural products: A review. Int. J. Green Pharm. 2016, 10, S96–S113. [Google Scholar]
- Tan, X.C.; Chua, K.H.; Ram, M.R.; Kuppusamy, U.R. Monoterpenes: Novel insights into their biological effects and roles on glucose uptake and lipid metabolism in 3T3-L1 adipocytes. Food Chem. 2016, 196, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Jelenkovic, L.; Jovanovic, V.; Palic, I.; Mitic, V.; Radulovic, M. In Vitro Screening of α-Amylase Inhibition by Selected Terpenes from Essential Oils. Trop. J. Pharm. Res. 2014, 13, 1421. [Google Scholar] [CrossRef] [Green Version]
- Sahin Basak, S.; Candan, F. Chemical composition and In vitro antioxidant and antidiabetic activities of Eucalyptus Camaldulensis Dehnh. essential oil. J. Iran Chem. Soc. 2010, 7, 216–226. [Google Scholar] [CrossRef]
- Enzymatic Assay of Alpha-Amylase Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/technical-documents/protocols/biology/enzymatic-assay-of-a-amylase.html (accessed on 20 August 2020).
- Bernfeld, P. Amylases, α and β. In Methods in Enzymology; Colowick, S.P., Kaplan, N., Eds.; Academic Press, Inc.: Cambridge, MA, USA; Harcourt Brace & Company: San Diego, CA, USA; New York, NY, USA; Boston, MA, USA; London, UK; Sydney, Australia; Tokyo, Japan; Toronto, ON, Canada, 1955; pp. 149–158. [Google Scholar]
- Rubiolo, P.; Sgorbini, B.; Liberto, E.; Cordero, C.; Bicchi, C. Essential oils and volatiles: Sample preparation and analysis. A review. Flavour. Fragr. J. 2010, 25, 282–290. [Google Scholar] [CrossRef]
- Bicchi, C.; Liberto, E.; Matteodo, M.; Sgorbini, B.; Mondello, L.; Zellner, B.; d’Acampora Costa, R.; Rubiolo, P. Quantitative analysis of essential oils: A complex task. Flavour. Fragr. J. 2008, 23, 382–391. [Google Scholar] [CrossRef]


| Species | Family | Common Name | Plant Part | % Inhibition Activity | Standard Deviation |
|---|---|---|---|---|---|
| Artemisia vulgaris L. | Compositae | Mugwort | Leaf/Flower | 48 | 3 |
| Cananga odorata (Lam.) Hook.f. and Thomson | Annonaceae | Ylang-ylang | Flower | no activity | - |
| Carum carvi L. | Apiaceae | Caraway | Seed | 17 | 2 |
| Cedrus atlantica (Endl.) Manetti ex Carrière | Pinaceae | Cedar wood | Wood | no activity | - |
| Chrysopogon zizanioides (L.) Roberty | Poaceae | Vetiver | Root | no activity | - |
| Cinnamomum zeylanicum Nees | Lauraceae | Cinnamon leaf | Leaf | no activity | - |
| Cinnamomum zeylanicum Nees | Lauraceae | Cinnamon bark | Bark | no activity | - |
| Cinnamomum camphora (L.) J.Presl | Lauraceae | Camphor | Wood | 20 | 2 |
| Cinnamomum cassia (L.) J.Presl | Lauraceae | Cinnamon bark | Bark | no activity | - |
| Citrus × aurantium L. | Rutaceae | Bitter orange | Fruit peel | 23 | 5 |
| Citrus × aurantium L. | Rutaceae | Neroli | Flower | 20 | 1 |
| Citrus × aurantium L. | Rutaceae | Petitgrain | Leaf | 4 | 1 |
| Citrus bergamia Risso et Poiteau | Rutaceae | Bergamot | Fruit peel | 16 | 3 |
| Citrus limon (L.) Osbeck | Rutaceae | Lemon | Fruit peel | 15 | 2 |
| Citrus medica L. | Rutaceae | Finger citron | Fruit peel | 14 | 4 |
| Citrus nobilis Lour. | Rutaceae | Mandarin | Fruit peel | no activity | - |
| Citrus paradisi Macfad. | Rutaceae | Grapefruit | Fruit peel | no activity | - |
| Citrus sinensis (L.) Osbeck | Rutaceae | Sweet orange | Fruit peel | 14 | 3 |
| Corymbia citriodora (Hook.) K.D.Hill and L.A.S.Johnson | Myrtaceae | Eucalyptus. lemon-scented | Leaf | 44 | 5 |
| Cupressus sempervirens L. | Cupressaceae | Cypress | Leaf/Twig | 17 | 1 |
| Cymbopogon martini (Roxb.) W.Watson. | Poaceae | Palmarosa | Leaf | no activity | - |
| Cymbopogon nardus (L.) Rendle | Poaceae | Citronella Ceylon | Leaf | 22 | 2 |
| Cymbopogon schoenanthus (L.) Spreng. | Poaceae | Lemongrass | Leaf | 7 | 2 |
| Elettaria cardamomum (L.) Maton | Zingiberaceae | Cardamom | Seed | 10 | 3 |
| Eucalyptus globulus Labill. | Myrtaceae | Eucalyptus | Leaf | 34 | 3 |
| Eucalyptus radiata A.Cunn. ex DC. | Myrtaceae | Leaf | 65 | 4 | |
| Foeniculum vulgare Mill. | Apiaceae | Fennel | Fruit | no activity | - |
| Gaultheria procumbens L. | Ericaceae | Wintergreen | Leaf | no activity | - |
| Hyssopus officinalis L. | Lamiaceae | Hyssop | Leaf | 18 | 1 |
| Jasminum officinale L. | Oleaceae | Jasmine | Flowers | no activity | - |
| Juniperus communis L. | Cupressaceae | Juniper berry | Fruit | 5 | 2 |
| Juniperus virginiana L. | Cupressaceae | Cedarwood | Wood | 15 | 2 |
| Laurus nobilis L. | Lauraceae | Laurel | Leaf | 50 | 4 |
| Lavandula angustifolia Mill. × L. latifolia Medik. | Lamiaceae | Lavandin | Leaf | 6 | 3 |
| Lavandula angustifolia Mill. | Lamiaceae | Lavender | Leaf | 5 | 2 |
| Matricaria chamomilla L. | Compositae | Chamomile | Flowers | 32 | 4 |
| Melaleuca alternifolia (Maiden and Betche) Cheel | Myrtaceae | Tea tree | Leaf | 14 | 2 |
| Melaleuca viridiflora Sol. ex Gaertn. | Myrtaceae | Niaouli | Leaf | 28 | 4 |
| Melissa officinalis L. | Lamiaceae | Lemon balm | Leaf | no activity | - |
| Mentha × piperita L. | Lamiaceae | Peppermint | Leaf | 33 | 3 |
| Mentha × piperita L. | Lamiaceae | Peppermint | Leaf/twig | 40 | 5 |
| Mentha arvensis L. | Lamiaceae | Mint | Leaf | 39 | 6 |
| Myristica fragrans Houtt. | Myristicaceae | Nutmeg | Seed | 59 | 4 |
| Myrtus communis L. | Myrtaceae | Myrtle | Leaf | 20 | 7 |
| Ocimum basilicum L. | Lamiaceae | Basil | Leaf | 14 | 5 |
| Origanum majorana L. | Lamiaceae | Marjoram | Leaf | 10 | 1 |
| Origanum vulgare L. | Lamiaceae | Oregano | Leaf | 9 | 4 |
| Pelargonium graveolens L’Hér. | Geraniaceae | Geranium | Leaf | 9 | 3 |
| Pimpinella anisum L. | Apiaceae | Aniseed | Fruit | 27 | 5 |
| Pinus mugo Turra | Pinaceae | Pine needle | Leaf/Twig | no activity | - |
| Pinus sylvestris L. | Pinaceae | Pine sylvestris | Leaf/Twig | no activity | - |
| Piper nigrum L. | Piperaceae | Black pepper | Fruit | no activity | - |
| Pogostemon cablin (Blanco) Benth. | Lamiaceae | Patchouli | Leaf | no activity | - |
| Rosa × damascena Herrm. | Rosaceae | Rosa | Flower | no activity | - |
| Salvia officinalis L. | Lamiaceae | Sage. Dalmatian | Leaf | 3 | 2 |
| Salvia sclarea L. | Lamiaceae | Clary sage | Leaf/Flower | no activity | - |
| Santalum album L. | Santalaceae | Sandalwood | Wood | no activity | - |
| Syzygium aromaticum (L.) Merr. and L.M.Perry | Myrtaceae | Clove oil | Leaf/Buds | no activity | - |
| Thuja occidentalis L. | Cupressaceae | Cedar leaf | Leaf | 7 | 5 |
| Thymus vulgaris L. | Lamiaceae | Thyme | Leaf | no activity | - |
| Verbena officinalis L. | Verbenaceae | Vervain | Leaf | no activity | - |
| Zingiber officinale Roscoe | Zingiberaceae | Ginger | Rhizome | no activity | - |
| % Inhibition Approach A | IC50 1 (mg mL−1) | % Inhibition Approach B | IC50 1 (mg mL−1) | % CV 2 | |
|---|---|---|---|---|---|
| Eucalyptus radiata | 65 ± 3 | 1.53 ± 0.08 | 65 ± 4 | 1.54 ± 0.11 | 0.080 |
| Myristica fragrans | 59 ± 4 | 1.70 ± 0.13 | 58 ± 5 | 1.71 ± 0.17 | 0.780 |
| Laurus nobilis | 51 ± 8 | 1.98 ± 0.32 | 51 ± 5 | 1.98 ± 0.19 | 0.120 |
| Acarbose | 56 ± 6 | 1.80 ± 0.20 | 55 ± 6 | 1.80 ± 0.22 | 0.020 |
| Species | Hydrocarbon Compounds | Oxygenated Compounds | List of the Most Abundant Components |
|---|---|---|---|
| Artemisia vulgaris L | 9.0 | 91.0 | α-Thujone (47.4), Camphor (30.0), β-Thujone (7.80), Sabinene (3.90), Camphene (3.70) |
| Carum carvi L. | 37.8 | 62.2 | Carvone (59.6), Limonene (35.4), β-Myrcene (0.700), cis-Dihydroxy carvone (0.600), trans-Dihydroxy carvone (0.200) |
| Cinnamomum camphora (L.) J.Presl | 54.8 | 45.2 | 1,8-Cineole (44.1), Limonene (17.4), p-Cymene (14.6), α-Terpinene (9.60), β-Pinene (7.60) |
| Citrus × aurantium L. (neroli) | 97.5 | 2.5 | Linalyl acetate (41.4), Linalool (28.5), Limonene (11.4), β-Pinene (7.60), trans-β-Ocimene (2.60) |
| Citrus × aurantium L. | 23.9 | 76.1 | Limonene (90.2), β -Myrcene (3.70), Linalyl acetate (1.60), α-Pinene (0.900), Sabinene (0.500) |
| Citrus × aurantium L. (Petit grain) | 7.4 | 92.6 | Linalyl acetate (56.8), Linalool (24.4), α-Terpineol (5.60), Geranyl acetate (3.40), Neryl acetate (1.80) |
| Citrus bergamia Risso et Poiteau | 52.0 | 48.0 | Linalyl acetate (34.1), Limonene (32.3), γ-Terpinene (7.80), β-Pinene (6.60), α-Pinene (1.00) |
| Citrus limon (L.) Osbeck | 97.2 | 2.8 | Limonene (71.9), β-Pinene (11.6), γ -Terpinene (7.90), α-Pinene (1.50), β-Myrcene (1.50) |
| Citrus medica L. | 72.5 | 27.5 | Limonene (54.9), Linalyl acetate (14.5), β-Pinene (9.10), Linalool (4.60), Geranial (4.60) |
| Citrus nobilis Lour. | 99.9 | 0.1 | Limonene (75.6), γ -Terpinene (14.5), α-Pinene (1.90), β-Pinene (1.10), β -Myrcene (1.00) |
| Corymbia citriodora (Hook.) K.D.Hill and L.A.S.Johnson | 1.8 | 98.2 | Citronellal (81.0), Neoisopulegol (7.10), Citronellol (6.00), Citronellyl acetate (1.50), 1,8-Cineole (0.800) |
| Cupressus sempervirens L. | 91.9 | 8.1 | α-Pinene (46.7), Δ-3-Carene (25.3), α-Terpinolene (4.20), Limonene (4.00), α-Terpinyl acetate (3.30) |
| Cymbopogon nardus (L.) Rendle | 11.6 | 88.4 | Citronellal (37.9), Geraniol (19.5), Citronellol (12.5), Limonene (7.20), Geranyl acetate (4.40) |
| Cymbopogon schoenanthus (L.) Spreng. | 7.0 | 93.0 | Geranial (38.2), Neral (32.5), Geraniol (7.30), Geranyl acetate (4.20), trans-β-Caryophyllene (2.90) |
| Elettaria cardamomum (L.) Maton | 9.2 | 90.8 | α-Terpinyl acetate (43.8), 1,8-Cineole (34.7), Linalyl acetate (6.00), Linalool (2.70), Limonene (2.20) |
| Eucalyptus globulus Labill. | 16.9 | 83.1 | 1,8-Cineole (82.1), Limonene (6.80), γ-Terpinene (3.30), p-Cymene (3.10), α-Pinene (2.20) |
| Eucalyptus radiata A.Cunn. ex DC. | 14.6 | 84.9 | 1,8-Cineole (75.1), α-Terpineol (7.6), Limonene (4.3) α-Terpinene (4.4) α-Pinene (2.7) |
| Hyssopus officinalis L. | 34.8 | 65.2 | 1,8-Cineole (39.2), α-Pinene (7.10), Isopinocamphone (6.10), Sabinene (5.80), β-Pinene (5.60) |
| Juniperus communis L. | 95.4 | 4.6 | α-Pinene (35.9), β -Myrcene (14.2), Sabinene (8.4) Limonene (8.00), β-Pinene (5.40) |
| Juniperus virginiana L. | 99.7 | 0.3 | β -Himachalene (50.8), α -Himalachene (16.0), γ-Himalachene (10.0), δ-Cadinene (2.50), α -Chamigrene (2.00) |
| Laurus nobilis L. | 20.5 | 79.5 | 1,8-Cineole (65.4), α-Terpinyl acetate (8.10), α-Pinene (6.4), Sabinene (5.10), β-Pinene (3.80) |
| Lavandula angustifolia Mill. × L. latifolia Medik. | 8.9 | 91.1 | Linalyl acetate (35.6), Linalool (27.1), Camphor (9.40), 1,8-Cineole (7.60), Borneol (3.40) |
| Lavandula angustifolia Mill. | 13.8 | 86.2 | Linalyl acetate (34.7), Linalool (27.9), trans-β-Caryophyllene (4.20), 4-Terpineol (3.70), Lavandulyl acetate (3.70) |
| Litsea cubeba (Lour.) Pers. | 17.3 | 82.7 | Geranial (42.4), Neral (34.6), Limonene (12.6), Sabinene (1.90), α-Pinene (1.20) |
| Matricaria chamomilla L. | 36.0 | 64.0 | α-Bisabolol oxide A (47.0), trans-β-Farnesene (24.0), α-Bisabolol oxide B (6.4), Chamazulene (2.60), Germacrene D (1.60) |
| Melaleuca alternifolia (Maiden and Betche) Cheel | 45.9 | 54.1 | 4-Terpineol (44.1), γ-Terpinene (21.1), α-Terpinene (9.40), α-Terpinolene (3.20), 1,8-Cineole (3.20) |
| Melaleuca viridiflora Sol. ex Gaertn. | 23.4 | 76.6 | 1,8-Cineole (64.9), Limonene (9.50), α-Pinene (6.20), α-Terpineol (4.20), Viridiflorol (2.40) |
| Mentha × piperita L. (leaf) | 1.1 | 98.9 | Menthol (48.2), Menthone (24.9), Isomenthone (13.3), Menthyl acetate (6.40), Neomenthol (2.00) |
| Mentha × piperita L. (Leaf/Twig) | 0.9 | 99.1 | Menthol (52.0), Menthone (23.0), Isomenthone (10.0), Menthyl acetate (4.60), Neomenthol (4.26) |
| Mentha arvensis L. | 6.6 | 93.4 | Menthol (40.2), Menthone (19.5), Isomenthone (8.00), Menthyl acetate (7.40), Neomenthol (5.20) |
| Myristica fragrans Houtt. | 85.2 | 14.8 | Sabinene (27.0), α-Pinene (23.0), β-Pinene (13.0), Limonene (10.0), 4-Terpineol (6.70) |
| Myrtus communis L. | 56.1 | 43.9 | Limonene (28.9), α-Pinene (15.1), Mirtenyl acetate (13.6), Linalool (13.50), Linalyl acetate (5.00) |
| Ocimum basilicum L | 5.1 | 94.9 | Estragole (88.4), 1,8-Cineole (3.40), α-trans-Bergamotene (2.30), trans-β-Ocimene (1.00), Linalool (0.600) |
| Origanum majorana L. | 41.3 | 58.7 | Linalool (34.0), 4-Terpineol (17.7), γ-Terpinene (10.8), α-Terpinene (7.00), Sabinene (5.80) |
| Origanum vulgare L. | 28.8 | 71.2 | Carvacrol (67.4), p-Cymene (12.2), γ-Terpinene (5.10), trans-β-Caryophyllene (4.70), Linalool (1.90) |
| Pelargonium graveolens L’Hér. | 5.1 | 94.9 | Citronellol (34.6), Geraniol (18.5), Citronellyl formate (9.50), Linalool (6.70), Isomenthone (5.10) |
| Pimpinella anisum L. | 3.7 | 96.3 | trans-Anethol (92.2), Limonene (2.1), Estragole (1.70), Foeniculin (.0700), Linalool (0.400) |
| Rosmarinus officinalis L. | 35.1 | 64.9 | 1,8-Cineole (43.3), Camphor (18.1), α-Pinene (12.8), β-Pinene (9.50), trans-β-Caryophyllene (5.90) |
| Salvia officinalis L. | 32.2 | 67.8 | α-Thujone (22.5), Camphor (18.5), 1,8-Cineole (11.4), α-Humulene (7.20), β-Thujone (6.20), |
| Syzygium aromaticum (L.) Merr. and L.M.Perry | 10.2 | 89.8 | Eugenol (82.0), trans-β-Caryophyllene (9.10), Eugenyl acetate (7.10), α-Humulene (1.10), Caryophyllene oxide (0.300) |
| # | Compounds | Eucalyptus radiata | Myristica fragrans | ||||
|---|---|---|---|---|---|---|---|
| Total EO | Hydrocarbon Fraction | Oxygenated Fraction | Total EO | Hydrocarbon Fraction | Oxygenated Fraction | ||
| 1 | α-Thujene | 0.2 | 0.3 | / | / | 0.7 | / |
| 2 | α-Pinene | 2.7 | 6.2 | / | 23.0 | 21.8 | / |
| 3 | Sabinene | 1.0 | 5.7 | / | 27.0 | 26.3 | / |
| 4 | β-Pinene | 0.6 | 4.2 | / | 13.0 | 20.4 | / |
| 5 | β-Mircene | 0.4 | 5.8 | / | 1.1 | 1.7 | / |
| 6 | α-Phellandrene | / | / | / | 1.4 | 1.6 | / |
| 7 | Δ-3-Carene | / | / | / | 0,4 | 0.6 | / |
| 8 | α-Terpinene | 4.4 | / | / | 0.9 | 1.2 | / |
| 9 | p-Cimene | 0.6 | 11.3 | / | 0.7 | 2.1 | / |
| 10 | Limonene | 4.3 | 65.4 | / | 10.0 | 15.3 | / |
| 11 | 1,8-Cineole | 75.1 | / | 69.8 | 1.8 | / | 5.0 |
| 12 | trans-β-Ocimene | tr | 0.2 | / | 0.05 | 0.1 | / |
| 13 | γ-Terpinene | tr | 0.1 | / | 4.9 | 6.6 | / |
| 14 | α-Terpinolene | tr | 0.1 | / | 0.6 | 1.1 | / |
| 15 | cis-Sabinene Hydrate | / | / | / | / | / | 0.7 |
| 16 | trans-Sabinene Hydrate | / | / | / | / | / | 0.5 |
| 17 | Linalool | 0.3 | / | 0.7 | 0,2 | / | 1.6 |
| 18 | Linalyl propionate | 0.2 | / | 0.4 | / | / | / |
| 19 | 4-Terpineol | 1.0 | / | 1.7 | 6.7 | / | 45.4 |
| 20 | α-Terpineol | 7.6 | / | 16.0 | 0.2 | / | 1.7 |
| 21 | Eugenol | / | / | / | 0.5 | / | 4.5 |
| 22 | Safrole | / | / | / | 0.7 | / | 1.1 |
| 23 | Myristicin | / | / | / | 4.4 | / | 37.3 |
| 24 | Neral | 0.3 | / | 1.2 | / | / | / |
| 25 | Geranial | 0.4 | / | 1.6 | / | / | / |
| 26 | α-Terpinyl acetate | 0.3 | / | 5.0 | / | / | 1.8 |
| 27 | trans-β-Caryophyllene | / | 0.6 | / | / | 0.2 | / |
| Repeatability (n = 3) | Intermediate Precision | ||||
|---|---|---|---|---|---|
| % Inhibition | % RSD | % Inhibition * | % RSD | ||
| Acarbose | 57 | 2 | Acarbose | 59 | 5 |
| 55 | 57 | ||||
| 56 | 53 | ||||
| 57 | |||||
| 55 | |||||
| 56 | |||||
| Laurel | 54 | 8 | Laurel | 54 | 12 |
| 51 | 53 | ||||
| 46 | 52 | ||||
| 56 | |||||
| 44 | |||||
| 42 | |||||
| Nutmeg | 56 | 4 | Nutmeg | 54 | 9 |
| 61 | 63 | ||||
| 60 | 60 | ||||
| 50 | |||||
| 65 | |||||
| 60 | |||||
| Eucalyptus | 66 | 4 | Eucalyptus | 54 | 9 |
| 68 | 70 | ||||
| 62 | 60 | ||||
| 62 | |||||
| 67 | |||||
| 69 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capetti, F.; Cagliero, C.; Marengo, A.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Bio-Guided Fractionation Driven by In Vitro α-Amylase Inhibition Assays of Essential Oils Bearing Specialized Metabolites with Potential Hypoglycemic Activity. Plants 2020, 9, 1242. https://doi.org/10.3390/plants9091242
Capetti F, Cagliero C, Marengo A, Bicchi C, Rubiolo P, Sgorbini B. Bio-Guided Fractionation Driven by In Vitro α-Amylase Inhibition Assays of Essential Oils Bearing Specialized Metabolites with Potential Hypoglycemic Activity. Plants. 2020; 9(9):1242. https://doi.org/10.3390/plants9091242
Chicago/Turabian StyleCapetti, Francesca, Cecilia Cagliero, Arianna Marengo, Carlo Bicchi, Patrizia Rubiolo, and Barbara Sgorbini. 2020. "Bio-Guided Fractionation Driven by In Vitro α-Amylase Inhibition Assays of Essential Oils Bearing Specialized Metabolites with Potential Hypoglycemic Activity" Plants 9, no. 9: 1242. https://doi.org/10.3390/plants9091242