Chitosan Modified Biochar Increases Soybean (Glycine max L.) Resistance to Salt-Stress by Augmenting Root Morphology, Antioxidant Defense Mechanisms and the Expression of Stress-Responsive Genes
Abstract
:1. Introduction
2. Materials and Methods
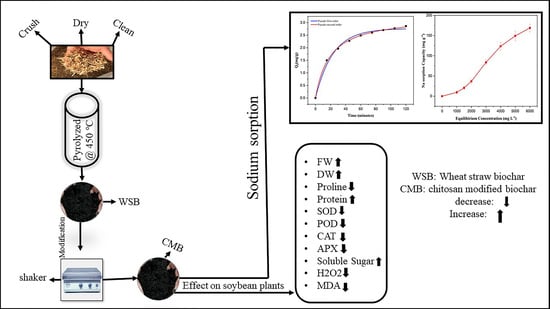
2.1. Characterization of Chitosan Modified Biochar
2.2. Plant Growth
2.3. Morphological Characteristics of Root and Shoot
2.4. Phosphorus and Nitrogen Contents Calculation
2.5. Measurement of Sodium Ion Concentration
2.6. Estimation of Total Protein, Soluble Sugar, and Chlorophyll Contents
2.7. Estimation of the Content of Proline and Glycine Betaine (GB)
2.8. Hydrogen Peroxide (H2O2) and Lipid Peroxidation (MDA) Measurement
2.9. Antioxidant Enzyme Assays
2.10. Total RNA Isolation and Analysis of Gene Expression Using Quantitative RT-PCR
2.11. Sodium Sorption of Chitosan Modified Biochar
2.12. Statistical Analysis
3. Results
3.1. Biochar Characteristics
3.1.1. Biochar pH and Elemental Composition
3.1.2. Biochar Surface Area, Porosity, Surface Functional Groups, and Surface Charge
3.2. Sorption Isotherm and Kinetics Study
3.3. Plant Growth and Biomass Yield
3.4. Phosphorus and Nitrogen Contents
3.5. Sodium-Ion Concentration
3.6. Soluble Protein, Soluble Sugar, and Chlorophyll Contents
3.7. Proline and Glycine Betaine Contents
3.8. Contents of H2O2 and MDA
3.9. Activities of Antioxidant Enzymes
3.10. Screening and Expression Analysis of Antioxidant Enzymes-Encoding Genes and Salt-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lam, H.M.; Xu, X.; Liu, X.; Chen, W.; Yang, G.; Wong, F.L.; Li, M.W.; He, W.; Qin, N.; Wang, B.; et al. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat. Genet. 2010, 42, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Han, X.; Zuo, J.F.; Song, J.; Han, C.Y.; Zhang, Y.W.; Zhang, Y.M. Identification of QTNs and their candidate genes for 100-seed weight in soybean (Glycine max L.) using multi-locus genome-wide association studies. Genes 2020, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Mageed, T.A.; Rady, M.M.; Taha, R.S.; Abd El Azeam, S.; Simpson, C.R.; Semida, W.M. Effects of integrated use of residual sulfur-enhanced biochar with effective microorganisms on soil properties, plant growth and short-term productivity of Capsicum annuum under salt stress. Sci. Hortic. 2020, 261, 108930. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Jha, A.B.; Dubey, R.S. Oxidative stress and antioxidative defense systems in plants growing under abiotic stresses. In Handbook of Plant and Crop Stress, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781439813997. [Google Scholar]
- Silberbush, M.; Ben-Asher, J.; Ephrath, J.E. A model for nutrient and water flow and their uptake by plants grown in a soilless culture. Plant Soil 2005, 271, 309–319. [Google Scholar] [CrossRef]
- Guo, R.; Yang, Z.; Li, F.; Yan, C.; Zhong, X.; Liu, Q.; Xia, X.; Li, H.; Zhao, L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, L.; Shen, Q.; Wu, L.; Yu, J.; Fu, L.; Wu, D.; Zhang, G. Identification of microRNAs responding to salt stress in barley by high-throughput sequencing and degradome analysis. Environ. Exp. Bot. 2019, 160, 59–70. [Google Scholar] [CrossRef]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014. [Google Scholar] [CrossRef]
- Drake, J.A.; Cavagnaro, T.R.; Cunningham, S.C.; Jackson, W.R.; Patti, A.F. Does Biochar Improve Establishment of Tree Seedlings in Saline Sodic Soils? L. Degrad. Dev. 2016, 27, 52–59. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ibrahim, M.; Zia-ur-Rehman, M.; Abbas, T.; Ok, Y.S. Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 2230–2248. [Google Scholar] [CrossRef]
- Morais, W.A.; de Almeida, A.L.P.; Pereira, M.R.; Fonseca, J.L.C. Equilibrium and kinetic analysis of methyl orange sorption on chitosan spheres. Carbohydr. Res. 2008, 343, 2489–2493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Q.; Hu, P.; Huang, R. Adsorptive removal of methyl orange using enhanced cross-linked chitosan/bentonite composite. Desalin. Water Treat. 2016, 57, 17011–17022. [Google Scholar] [CrossRef]
- Yuvaraja, G.; Su, M.; Chen, D.Y.; Pang, Y.; Kong, L.J.; Subbaiah, M.V.; Wen, J.C.; Reddy, G.M. Impregnation of magnetic—Momordica charantia leaf powder into chitosan for the removal of U(VI) from aqueous and polluted wastewater. Int. J. Biol. Macromol. 2020, 149, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, G.; Shao, H.B. Furfural and its biochar improve the general properties of a saline soil. Solid Earth 2014, 5, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Liu, G.; Xia, Y.; Chen, L.; Jiang, Z.; Zheng, H.; Wang, Z. Use of biochar-compost to improve properties and productivity of the degraded coastal soil in the Yellow River Delta, China. J. Soils Sediments 2017, 17, 780–789. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, D.; Bai, Y.; Wang, N.; Wang, Y. Studies on the overexpression of the soybean GmNHX1 in Lotus corniculatus: The reduced Na+ level is the basis of the increased salt tolerance. Chin. Sci. Bull. 2006, 51, 1306–1315. [Google Scholar] [CrossRef]
- Pardo, J.M.; Cubero, B.; Leidi, E.O.; Quintero, F.J. Alkali cation exchangers: Roles in cellular homeostasis and stress tolerance. Proc. J. Exp. Bot. 2006, 57, 1181–1199. [Google Scholar] [CrossRef] [Green Version]
- Guan, R.; Qu, Y.; Guo, Y.; Yu, L.; Liu, Y.; Jiang, J.; Chen, J.; Ren, Y.; Liu, G.; Tian, L.; et al. Salinity tolerance in soybean is modulated by natural variation in GmSALT3. Plant J. 2014, 80, 937–950. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.Z.; Wang, H.W.; Huang, J.; Tian, A.G.; Wang, Y.J.; Zhang, J.S.; Chen, S.Y. A putative plasma membrane cation/proton antiporter from soybean confers salt tolerance in Arabidopsis. Plant Mol. Biol. 2005, 59, 809–820. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Kaufman, R.C.; Wilson, J.D.; Shi, Y.C. Position of modifying groups on starch chains of octenylsuccinic anhydride-modified waxy maize starch. Food Chem. 2014, 153, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Saeed, D.A.; Rizwan, M.; Khan, M.N.; Aziz, O.; Bashir, S.; Ibrahim, M.; Ditta, A.; Akmal, M.; Mumtaz, M.A.; et al. Impact of different amendments on biochemical responses of sesame (Sesamum indicum L.) plants grown in lead-cadmium contaminated soil. Plant Physiol. Biochem. 2018, 132, 345–355. [Google Scholar] [CrossRef]
- Ahmad, M.; Ok, Y.S.; Rajapaksha, A.U.; Lim, J.E.; Kim, B.Y.; Ahn, J.H.; Lee, Y.H.; Al-Wabel, M.I.; Lee, S.E.; Lee, S.S. Lead and copper immobilization in a shooting range soil using soybean stover- and pine needle-derived biochars: Chemical, microbial and spectroscopic assessments. J. Hazard. Mater. 2016, 301, 179–186. [Google Scholar] [CrossRef]
- Hossain, Z.; Hajika, M.; Komatsu, S. Comparative proteome analysis of high and low cadmium accumulating soybeans under cadmium stress. Amino Acids 2012, 43, 2393–2416. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Peijnenburg, W.J.G.M.; Zhao, J.; Chen, X.; Yu, J.; Wu, H. Pathways of cadmium fluxes in the root of the halophyte Suaeda salsa. Ecotoxicol. Environ. Saf. 2012, 75, 1–7. [Google Scholar] [CrossRef]
- El Naim, A.M.; Mohammed, K.E.; Ibrahim, E.A.; Suleiman, N.N. Impact of Salinity on Seed Germination and Early Seedling Growth of Three Sorghum (Sorghum biolor L. Moench) Cultivars. Sci. Technol. 2012, 2, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Jiang, D.; Coles, N.; Wu, J. Effects of biochar on the acidity of a loamy clay soil under different incubation conditions. J. Soils Sediments 2015, 15, 1919–1926. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, B.; Zimmerman, A.R.; Fang, J.; Sun, Y.; Cao, X. Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chem. Eng. J. 2013, 231, 512–518. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 595–624. ISBN 0-89118-825-8. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Ning, L.; Kan, G.; Shao, H.; Yu, D. Physiological and transcriptional responses to salt stress in salt-tolerant and salt-sensitive soybean (Glycine max [L.] Merr.) seedlings. L. Degrad. Dev. 2018, 29, 2707–2719. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rastall, R.A. Methods in plant biochemistry. volume 2 carbohydrates edited by P. M. Dey, Academic Press, London, 1990, pp. ix + 675, price £79.00; US$149.0. ISBN 0 12 461012 9. J. Chem. Technol. Biotechnol. 2007, 52, 588–589. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Spinach chloroplasts scavenge hydrogen peroxide on illumination. Plant Cell Physiol. 1980, 21, 1295–1307. [Google Scholar] [CrossRef]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R. Methods of Enzymatic Analysis. J. Clin. Pathol. 1987, 40, 934. [Google Scholar] [CrossRef] [Green Version]
- Sirhindi, G.; Mir, M.A.; Abd-Allah, E.F.; Ahmad, P.; Gucel, S. Jasmonic acid modulates the physio-biochemical attributes, antioxidant enzyme activity, and gene expression in glycine max under nickel toxicity. Front. Plant Sci. 2016, 7, 1630. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alamri, S.A.; Ali, H.M.; Alayafi, A.A. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by modulating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol. Biochem. 2018, 132, 375–384. [Google Scholar] [CrossRef]
- Kim, J.H.; Seo, W.D.; Lee, S.K.; Lee, Y.B.; Park, C.H.; Ryu, H.W.; Lee, J.H. Comparative assessment of compositional components, antioxidant effects, and lignan extractions from Korean white and black sesame (Sesamum indicum L.) seeds for different crop years. J. Funct. Foods 2014, 7, 495–505. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Gu, H.; Wu, B.; Zhang, H.; Yuan, X.; Cui, X. GmHKT1;4, a novel soybean gene regulating Na+/K+ ratio in roots enhances salt tolerance in transgenic plants. Plant Growth Regul. 2014, 73, 299–308. [Google Scholar] [CrossRef]
- Zhou, G.A.; Guan, R.X.; Li, Y.H.; Chang, R.Z.; Qiu, L.J. Molecular characterization of GmNHX2, a Na+/H+ antiporter gene homolog from soybean, and its heterologous expression to improve salt tolerance in arabidopsis. Chin. Sci. Bull. 2009, 54, 3536–3545. [Google Scholar] [CrossRef] [Green Version]
- Phang, T.H.; Shao, G.; Liao, H.; Yan, X.; Lam, H.M. High external phosphate (Pi) increases sodium ion uptake and reduces salt tolerance of “Pi-tolerant” soybean. Physiol. Plant. 2009, 412–425. [Google Scholar] [CrossRef]
- Ifthikar, J.; Jiao, X.; Ngambia, A.; Wang, T.; Khan, A.; Jawad, A.; Xue, Q.; Liu, L.; Chen, Z. Facile One-Pot Synthesis of Sustainable Carboxymethyl Chitosan—Sewage Sludge Biochar for Effective Heavy Metal Chelation and Regeneration. Bioresour. Technol. 2018, 262, 22–31. [Google Scholar] [CrossRef]
- Cui, X.; Dai, X.; Khan, K.Y.; Li, T.; Yang, X.; He, Z. Removal of phosphate from aqueous solution using magnesium-alginate/chitosan modified biochar microspheres derived from Thalia dealbata. Bioresour. Technol. 2016, 218, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, H.; Ren, H. Dissolved organic matter (DOM) removal from biotreated coking wastewater by chitosan-modified biochar: Adsorption fractions and mechanisms. Bioresour. Technol. 2020, 297, 122281. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, B.; Zhou, D.; Chen, W. Bisolute sorption and thermodynamic behavior of organic pollutants to biomass-derived biochars at two pyrolytic temperatures. Environ. Sci. Technol. 2012, 46, 12476–12483. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wei, C.; Zhang, S.; Wang, Y.; Kuzyakov, Y.; Ding, X. MgO-modified biochar increases phosphate retention and rice yields in saline-alkaline soil. J. Clean. Prod. 2019, 235, 901–909. [Google Scholar] [CrossRef]
- Chen, H.; He, H.; Yu, D. Overexpression of a novel soybean gene modulating Na+ and K+ transport enhances salt tolerance in transgenic tobacco plants. Physiol. Plant. 2011, 141, 11–18. [Google Scholar] [CrossRef]
- Kausar, F.; Shahbaz, M.; Ashraf, M. Protective role of foliar-applied nitric oxide in Triticum aestivum under saline stress. Turk. J. Bot. 2013, 37, 1155–1165. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Chaoxing, H. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Chaoxing, H. Does Inoculation with Glomus mosseae Improve Salt Tolerance in Pepper Plants? J. Plant Growth Regul. 2014, 33, 644–653. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Fujita, M.; Tran, L.S.P. Nitric oxide mediates hydrogen peroxide- and salicylic acid-induced salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul. 2015, 77, 265–277. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The role of endogenous nitric oxide in salicylic acid-induced up-regulation of ascorbate-glutathione cycle involved in salinity tolerance of pepper (Capsicum annuum L.) plants. Plant Physiol. Biochem. 2020, 147, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bai, Z.; Huang, J.; Hussain, S.; Zhao, F.; Zhu, C.; Zhu, L.; Cao, X.; Jin, Q. Biochar alleviated the salt stress of induced saline paddy soil and improved the biochemical characteristics of rice seedlings differing in salt tolerance. Soil Tillage Res. 2019, 195, 104372. [Google Scholar] [CrossRef]
- Turan, V. Confident performance of chitosan and pistachio shell biochar on reducing Ni bioavailability in soil and plant plus improved the soil enzymatic activities, antioxidant defense system and nutritional quality of lettuce. Ecotoxicol. Environ. Saf. 2019, 183, 109594. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, W.Y.; Yun, D.J. A new insight of salt stress signaling in plant. Mol. Cells 2016. [Google Scholar] [CrossRef] [PubMed]
- Hammer, E.C.; Forstreuter, M.; Rillig, M.C.; Kohler, J. Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl. Soil Ecol. 2015, 96, 114–121. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, K.R.; Yang, J.E.; Ok, Y.S.; Owens, G.; Nehls, T.; Wessolek, G.; Kim, K.H. Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere 2016, 142, 153–159. [Google Scholar] [CrossRef]
- Turan, S.; Tripathy, B.C. Salt and genotype impact on antioxidative enzymes and lipid peroxidation in two rice cultivars during de-etiolation. Protoplasma 2013, 250, 209–222. [Google Scholar] [CrossRef]
- Alqarawi, A.; Hashem, A.; Abd Allah, E.; Alshahrani, T.; Huqail, A. Effect of salinity on moisture content, pigment system, and lipid composition in Ephedra alata Decne. Acta Biol. Hung. 2014, 65, 61–71. [Google Scholar] [CrossRef]
- Taïbi, K.; Taïbi, F.; Ait Abderrahim, L.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Cao, K.F. Gas exchange, chlorophyll fluorescence, and osmotic adjustment in two mango cultivars under drought stress. Acta Physiol. Plant. 2008, 30, 769–777. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Miransari, M. The role of arbuscular mycorrhizal fungi in alleviation of salt stress. In Use of Microbes for the Alleviation of Soil Stresses: Volume 2: Alleviation of Soil Stress by Pgpr and Mycorrhizal Fungi; Springer: New York, NY, USA, 2014; ISBN 9781493907212. [Google Scholar]
- Gupta, A.K.; Kaur, N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 2005, 30, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Phang, T.H.; Shao, G.; Lam, H.M. Salt tolerance in soybean. J. Integr. Plant Biol. 2008, 193, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Khan, E.A. Shahjahan Adsorptive uptake of basic dyes from aqueous solution by novel brown linseed deoiled cake activated carbon: Equilibrium isotherms and dynamics. J. Environ. Chem. Eng. 2016, 4, 3084–3095. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta—Bioenerg. 2010, 1797, 1428–1438. [Google Scholar] [CrossRef] [Green Version]
- Arshad, M.A.; Mir, A.K.; Mushtaq, A.; Mamoona, M.; Muhammad, Z.; Shazai, S.; Zia-u-Rehman, M.; Zahid, U. Ethnobotanical and taxonomic screening of genus Morus for wild edible fruits used by the inhabitants of Lesser Himalayas-Pakistan. J. Med. Plants Res. 2014, 8, 889–898. [Google Scholar] [CrossRef]
- Yen, W.J.; Chyau, C.C.; Lee, C.P.; Chu, H.L.; Chang, L.W.; Duh, P. Der Cytoprotective effect of white tea against H2O2-induced oxidative stress in vitro. Food Chem. 2013, 141, 4107–4114. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Biochar Increased Plant Growth-Promoting Hormones and Helped to Alleviates Salt Stress in Common Bean Seedlings. J. Plant Growth Regul. 2018, 37, 591–601. [Google Scholar] [CrossRef]
- Naeem, M.A.; Shabbir, A.; Amjad, M.; Abbas, G.; Imran, M.; Murtaza, B.; Tahir, M.; Ahmad, A. Acid treated biochar enhances cadmium tolerance by restricting its uptake and improving physio-chemical attributes in quinoa (Chenopodium quinoa Willd.). Ecotoxicol. Environ. Saf. 2020, 191, 110218. [Google Scholar] [CrossRef]
- Abd-Allah, E.F.; Hashem, A.; Alqarawi, A.A.; Bahkali, A.H.; Alwhibi, M.S. Enhancing growth performance and systemic acquired resistance of medicinal plant Sesbania sesban (L.) Merr using arbuscular mycorrhizal fungi under salt stress. Saudi J. Biol. Sci. 2015, 22, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.K.; Ahmaruzzaman, M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- Arumugaperumal, M.; Sujatha, K.B. Response of antioxidant enzymes in tomato genotypes exposed to elevated temperature. J. Pharmacogn. Phytochem. 2019, 2, 804–809. [Google Scholar]









| Treatments | P Content | N Content | Root Length | Shoot Length | Root FW | Shoot FW | Root DW | Shoot DW |
|---|---|---|---|---|---|---|---|---|
| % | cm | g | ||||||
| T1 | 0.3 ± 0.02 BCD | 0.8 ± 0.06 CD | 12 ± 0.91 BC | 19 ± 1.46 CD | 0.9 ± 0.07 B | 1.4 ± 0.1 B | 0.10 ± 0.01 C | 0.27 ± 0.02 B |
| T2 | 0.4 ± 0.03 B | 0.8 ± 0.06 CD | 13 ± 0.99 B | 24 ± 1.8 B | 0.9 ± 0.07 B | 1.5 ± 0.12 B | 0.12 ± 0.01 B | 0.28 ± 0.02 B |
| T3 | 0.5 ± 0.04 A | 1.1 ± 0.09 A | 20 ± 1.52 A | 32 ± 2.45 A | 1.4 ± 0.11 A | 2.1 ± 0.16 A | 0.16 ± 0.01 A | 0.42 ± 0.03 A |
| T4 | 0.3 ± 0.03 BC | 0.7 ± 0.06 D | 8 ± 0.69 E | 15 ± 1.23 FG | 0.6 ± 0.05 C | 0.8 ± 0.07 D | 0.05 ± 0 E | 0.18 ± 0.02 C |
| T5 | 0.3 ± 0.03 BC | 0.8 ± 0.07 BC | 11 ± 0.82 CD | 17 ± 1.33 DEF | 0.7 ± 0.05 C | 1.1 ± 0.08 C | 0.06 ± 0 E | 0.21 ± 0.02 C |
| T6 | 0.4 ± 0.03 B | 0.9 ± 0.07 B | 13 ± 1.03 B | 20 ± 1.62 C | 0.7 ± 0.06 C | 1.1 ± 0.09 C | 0.08 ± 0.01 D | 0.20 ± 0.02 C |
| T7 | 0.3 ± 0.02 E | 0.5 ± 0.04 E | 5 ± 0.42 F | 14 ± 1.1 G | 0.4 ± 0.03 D | 0.6 ± 0.05 E | 0.03 ± 0 F | 0.08 ± 0.01 D |
| T8 | 0.2 ± 0.02 DE | 0.5 ± 0.04 E | 7 ± 0.6 E | 16 ± 1.35 EFG | 0.5 ± 0.04 D | 0.7 ± 0.06 DE | 0.04 ± 0 F | 0.08 ± 0.01 D |
| T9 | 0.33 ± 0.03 E | 0.6 ± 0.05 E | 9 ± 0.78 D | 19 ± 1.46 CDE | 0.7 ± 0.05 C | 0.9 ± 0.07 D | 0.05 ± 0 E | 0.10 ± 0.01 D |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehmood, S.; Ahmed, W.; Ikram, M.; Imtiaz, M.; Mahmood, S.; Tu, S.; Chen, D. Chitosan Modified Biochar Increases Soybean (Glycine max L.) Resistance to Salt-Stress by Augmenting Root Morphology, Antioxidant Defense Mechanisms and the Expression of Stress-Responsive Genes. Plants 2020, 9, 1173. https://doi.org/10.3390/plants9091173
Mehmood S, Ahmed W, Ikram M, Imtiaz M, Mahmood S, Tu S, Chen D. Chitosan Modified Biochar Increases Soybean (Glycine max L.) Resistance to Salt-Stress by Augmenting Root Morphology, Antioxidant Defense Mechanisms and the Expression of Stress-Responsive Genes. Plants. 2020; 9(9):1173. https://doi.org/10.3390/plants9091173
Chicago/Turabian StyleMehmood, Sajid, Waqas Ahmed, Muhammad Ikram, Muhammad Imtiaz, Sammina Mahmood, Shuxin Tu, and Diyun Chen. 2020. "Chitosan Modified Biochar Increases Soybean (Glycine max L.) Resistance to Salt-Stress by Augmenting Root Morphology, Antioxidant Defense Mechanisms and the Expression of Stress-Responsive Genes" Plants 9, no. 9: 1173. https://doi.org/10.3390/plants9091173