Verticillium Wilt in Oilseed Rape—the Microbiome is Crucial for Disease Outbreaks as Well as for Efficient Suppression
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection Step One: Soil-Free In Planta Tests for a Preliminary Selection of Suitable Verticillium Antagonists
2.2. Selection Step Two: Soil-Free In Planta Tests for a Selection of Verticillium Antagonistic Consortia
2.3. Selection Step Three: In Situ Tests in Soil for a Selection of Suitable Verticillium Antagonists
2.4. Evaluation Step One: Integrating Different Cultivars
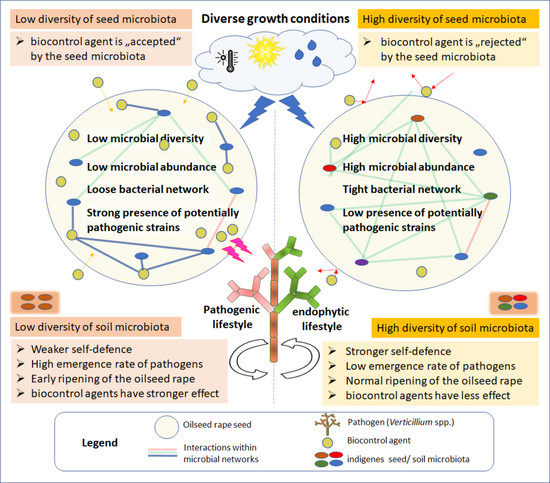
2.5. Evaluation Step Two: Integrating Seed Microbiome Studies
2.6. Evaluation Step Three: Analyzing Biocontrol of Verticillium Wilt under Field Conditions
2.7. Evaluation Step Four: Visualization of the Interaction between the host plant, Verticillium and its Bacterial Counterpart using Confocal Laser Scanning Microscopy (CLSM)
2.8. Assessment: Analyzing the Ecological Footprint
2.9. Assessment: Analyzing the Ecological Background
3. Material and Methods
3.1. Bacterial Strains and Growth Conditions
3.2. Co-Inoculation Studies under Sterile Soil-Free Conditions
3.3. Field Trials
3.4. Visualization of the Bacterial and Fungal Interaction Ad Planta using CLSM
3.5. Assessment of Ecological Footprint of the Treatment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karapapa, V.K.; Bainbridge, B.W.; Heale, J.B. Morphological and molecular characterization of Verticillium longisporum comb, nov., pathogenic to oilseed rape. Mycol. Res. 1997, 101, 1281–1294. [Google Scholar] [CrossRef]
- Stark, C. Das Auftreten der Verticillium-Tracheomykosen in Hamburger Gartenbaukulturen: Ein Beitrag zur Kenntnis ihrer Erreger. Gartenbauwissenschaft 1961, 26, 493–528. [Google Scholar]
- Gladders, P.; Smith, J.; Kirkpatrick, L.; Clewes, E.; Grant, C.; Barbara, D.J.; Barnes, A.; Lane, C. First record of verticillium wilt (Verticillium longisporum) in winter oilseed rape in the UK. New Dis. Rep. 2011, 23, 8. [Google Scholar] [CrossRef] [Green Version]
- Steventon, L.A.; Fahleson, J.; Qiong, H.; Dixelius, C. Identification of the causal agent of Verticillium wilt of winter oilseed rape in Sweden, V. longisporum. Mycol. Res. 2002, 106, 570–578. [Google Scholar] [CrossRef]
- Zeise, K.; Von Tiedemann, A. Application of RAPD-PCR for Virulence Type Analysis within Verticillium dahliae and V. longisporum. J. Phytopathol. 2002, 150, 557–563. [Google Scholar] [CrossRef]
- Pantou, M.P.; Strunnikova, O.K.; Shakhnazarova, V.Y.; Vishnevskaya, N.A.; Papalouka, V.G.; Typas, M.A. Molecular and immunochemical phylogeny of Verticillium species. Mycol. Res. 2005, 109, 889–902. [Google Scholar] [CrossRef]
- Hwang, S.-F.; Strelkov, S.E.; Ahmed, H.U.; Zhou, Q.; Fu, H.; Fredua-Agyeman, R.; Turnbull, G.D. First report of Verticillium dahliae Kleb. causing wilt symptoms in canola (Brassica napus L.) in North America. Can. J. Plant Pathol. 2017, 39, 514–526. [Google Scholar] [CrossRef]
- Inderbitzin, P.; Davis, R.M.; Bostock, R.M.; Subbarao, K.V. The ascomycete Verticillium longisporum is a hybrid and a plant pathogen with an expanded host range. PLoS ONE 2011, 6(3), e18260. [Google Scholar] [CrossRef] [Green Version]
- Novakazi, F.; Inderbitzin, P.; Sandoya, G.; Hayes, R.J.; von Tiedemann, A.; Subbarao, K.V. The three lineages of the diploid hybrid Verticillium longisporum differ in virulence and pathogenicity. Phytopathology 2015, 105, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Rafiei, V.; Banihashemi, Z.; Jiménez-Díaz, R.M.; Navas-Cortés, J.A.; Landa, B.B.; Jiménez-Gasco, M.M.; Turgeon, B.G.; Milgroom, M.G. Comparison of genotyping by sequencing and microsatellite markers for unravelling population structure in the clonal fungus Verticillium dahliae. Plant Pathol. 2018, 67, 76–86. [Google Scholar] [CrossRef]
- Jiménez-Díaz, R.M.; Olivares-García, C.; Trapero-Casas, J.L.; Jiménez-Gasco, M.; Navas-Cortés, J.A.; Landa, B.B.; Milgroom, M. Variation of pathotypes and races and their correlations with clonal lineages in Verticillium dahliae. Plant Pathol. 2017, 66, 651–666. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Dung, J.K.S.; Johnson, D.A. From pathogen to endophyte: An endophytic population of Verticillium dahliae evolved from a sympatric pathogenic population. New Phytol. 2019, 222, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, C.; Green, K.J. Studies of the host specificity of Verticillium albo-atrum var. menthae. Phytopathology 1960, 50(9), 635. [Google Scholar]
- Woolliams, G. Host range and symptomatology of Verticillium dahliae in economic, weed, and native plants in interior British Columbia. Can. J. Plant Sci. 1966, 46, 661–669. [Google Scholar] [CrossRef]
- Malcolm, G.M.; Kuldau, G.A.; Gugino, B.K.; Jiménez-Gasco, M.d.M. Hidden host plant associations of soilborne fungal pathogens: An ecological perspective. Phytopathology 2013, 103, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Tyvaert, L.; França, S.; Debode, J.; Höfte, M. The endophyte Verticillium V t305 protects cauliflower against Verticillium wilt. J. Appl. Microbiol. 2014, 116, 1563–1571. [Google Scholar] [CrossRef]
- Elgersma, D.; Roosien, T.; Scheffer, R. Biological control of Dutch elm disease by exploiting resistance in the host. In Dutch Elm Disease Research; Springer: New York, NY, USA, 1993; pp. 188–192. [Google Scholar]
- Postma, J.; Goossen-van de Geijn, H. Twenty-four years of Dutch Trig® application to control Dutch elm disease. BioControl 2016, 61, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Wassermann, B.; Adam, E.; Cernava, T.; Berg, G. Understanding the Indigenous Seed Microbiota to Design Bacterial Seed Treatments. In Seed Endophytes: Biology and Biotechnology; Verma, S.K., White, J.F., Jr., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 83–99. ISBN 978-3-030-10504-4. [Google Scholar]
- Depotter, J.R.L.; Thomma, B.P.H.J.; Wood, T.A. Variable impact of Verticillium longisporum on oilseed rape yield in field trials in the United Kingdom. bioRxiv 2017, 205401. [Google Scholar] [CrossRef] [Green Version]
- Depotter, J.R.L.; Thomma, B.P.H.J.; Wood, T.A. Measuring the impact of Verticillium longisporum on oilseed rape (Brassica napus) yield in field trials in the United Kingdom. Eur. J. Plant Pathol. 2019, 153, 321–326. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT. Statistical Databases; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- Gunstone, F.D. Rapeseed and Canola Oil: Production, Processing, Properties and Uses; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bennett, A.J.; Bending, G.D.; Chandler, D.; Hilton, S.; Mills, P. Meeting the demand for crop production: The challenge of yield decline in crops grown in short rotations. Biol. Rev. Camb. Philos. Soc. 2012, 87, 52–71. [Google Scholar] [CrossRef]
- Hilton, S.; Bennett, A.J.; Keane, G.; Bending, G.D.; Chandler, D.; Stobart, R.; Mills, P. Impact of shortened crop rotation of oilseed rape on soil and rhizosphere microbial diversity in relation to yield decline. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Peralta, A.L.; Sun, Y.; McDaniel, M.D.; Lennon, J.T. Crop rotational diversity increases disease suppressive capacity of soil microbiomes. Ecosphere 2018, 9, e02235. [Google Scholar] [CrossRef]
- McDaniel, M.; Tiemann, L.; Grandy, A. Does agricultural crop diversity enhance soil microbial biomass and organic matter dynamics? A meta-analysis. Ecol. Appl. 2014, 24, 560–570. [Google Scholar] [CrossRef] [Green Version]
- Berg, G.; Köberl, M.; Rybakova, D.; Müller, H.; Grosch, R.; Smalla, K. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef]
- Depotter, J.R.L.; Deketelaere, S.; Inderbitzin, P.; Tiedemann, A.V.; Höfte, M.; Subbarao, K.V.; Wood, T.A.; Thomma, B.P.H.J. Verticillium longisporum, the invisible threat to oilseed rape and other brassicaceous plant hosts. Mol. Plant Pathol. 2016, 17, 1004–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcinkevičien, A.; Velička, R.; Butkevičien, L.M.; Keidan, M.; Pupalienė, R.; Kriaučiūnienė, Z.; Kosteckas, R.; Čekanauskas, S.; Raudonius, S. The impact of non-chemical weed control methods on the disease occurrence in the organically grown winter oilseed rape crop. Zemdirbyste-Agriculture 2018, 105, 331–338. [Google Scholar]
- Fradin, E.F.; Thomma, B.P. Physiology and molecular aspects of verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef]
- Song, Y.; Liu, L.; Wang, Y.; Valkenburg, D.; Zhang, X.; Zhu, L.; Thomma, B.P. Transfer of tomato immune receptor Ve1 confers Ave1-dependent Verticillium resistance in tobacco and cotton. Plant Biotechnol. J. 2018, 16, 638–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef]
- Research and Markets Biological Seed Treatment—Market Analysis, Trends, and Forecasts. Available online: https://www.researchandmarkets.com/reports/4804728/biological-seed-treatment-market-analysis (accessed on 31 March 2020).
- Nejad, P.; Johnson, P.A. Endophytic bacteria induce growth promotion and wilt disease suppression in oilseed rape and tomato. Biol. Control 2000, 18, 208–215. [Google Scholar] [CrossRef]
- Kalbe, C.; Marten, P.; Berg, G. Strains of the genus Serratia as beneficial rhizobacteria of oilseed rape with antifungal properties. Microbiol. Res. 1996, 151, 433–439. [Google Scholar] [CrossRef]
- Fiddaman, P.J.; Rossall, S. Selection of bacterial antagonists for the biological control of Rhizoctonia solani in oilseed rape (Brassica napus). Plant Pathol. 1995, 44, 695–703. [Google Scholar] [CrossRef]
- Gao, X.; Han, Q.; Chen, Y.; Qin, H.; Huang, L.; Kang, Z. Biological control of oilseed rape Sclerotinia stem rot by Bacillus subtilis strain Em7. Biocontrol Sci. Technol. 2014, 24, 39–52. [Google Scholar] [CrossRef]
- Alström, S. Characteristics of Bacteria from Oilseed Rape in Relation to their Biocontrol Activity against Verticillium dahliae. J. Phytopathol. 2001, 149, 57–64. [Google Scholar] [CrossRef]
- Müller, H.; Berg, G. Impact of formulation procedures on the effect of the biocontrol agent Serratia plymuthica HRO-C48 on verticillium wilt in oilseed rape. BioControl 2008, 53, 905–916. [Google Scholar] [CrossRef]
- Wassermann, B.; Rybakova, D.; Müller, C.; Berg, G. Harnessing the microbiomes of Brassica vegetables for health issues. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybakova, D.; Rack-Wetzlinger, U.; Cernava, T.; Schaefer, A.; Schmuck, M.; Berg, G. Aerial warfare: A volatile dialogue between the plant pathogen Verticillium longisporum and its antagonist Paenibacillus polymyxa. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Rybakova, D.; Schmuck, M.; Wetzlinger, U.; Varo-Suarez, A.; Murgu, O.; Müller, H.; Berg, G. Kill or cure? The interaction between endophytic Paenibacillus and Serratia strains and the host plant is shaped by plant growth conditions. Plant Soil 2016, 405, 65–79. [Google Scholar] [CrossRef]
- Rybakova, D.; Mancinelli, R.; Wikström, M.; Birch-Jensen, A.-S.; Postma, J.; Ehlers, R.-U.; Goertz, S.; Berg, G. The structure of the Brassica napus seed microbiome is cultivar-dependent and affects the interactions of symbionts and pathogens. Microbiome 2017, 5, 104. [Google Scholar] [CrossRef]
- Chew, F.S. Biological Effects of Glucosinolates. In Biologically Active Natural Products; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1988; Volume 380, pp. 155–181. ISBN 978-0-8412-1556-6. [Google Scholar]
- Berg, G.; Roskot, N.; Steidle, A.; Eberl, L.; Zock, A.; Smalla, K. Plant-dependent genotypic and phenotypic diversity of antagonistic rhizobacteria isolated from different Verticillium host plants. Appl. Environ. Microbiol. 2002, 68, 3328–3338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, G.; Opelt, K.; Zachow, C.; Lottmann, J.; Götz, M.; Costa, R.; Smalla, K. The rhizosphere effect on bacteria antagonistic towards the pathogenic fungus Verticillium differs depending on plant species and site. FEMS Microbiol. Ecol. 2006, 56, 250–261. [Google Scholar] [CrossRef]
- Rybakova, D.; Cernava, T.; Köberl, M.; Liebminger, S.; Etemadi, M.; Berg, G. Endophytes-assisted biocontrol: Novel insights in ecology and the mode of action of Paenibacillus. Plant Soil 2016, 405, 125–140. [Google Scholar] [CrossRef]
- Rybakova, D.; Wetzlinger, U.; Müller, H.; Berg, G. Complete genome sequence of Paenibacillus polymyxa strain Sb3-1, a soilborne bacterium with antagonistic activity toward plant pathogens. Genome Announc. 2015, 3, e00052-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ram, R.M.; Singh, H. Microbial consortium in biological control: An explicit example of teamwork below ground. J. Eco-Friendly Agric. 2018, 13, 1–12. [Google Scholar]
- Woo, S.L.; Pepe, O. Microbial Consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Berg, G. RhizoStar®: Ein biologisches Präparat für die Erdbeere auf der Basis des Wurzelbakteriums Serratia plymuthica HRO-C48. Obstbau 2003, 12, 34–36. [Google Scholar]
- Berg, G.; Kurze, S.; Dahl, R. Rhizobakerienisolate zur Anwendung gegen Phytopathogene Bodenpilze und Verfahren zur Anwendung der Rhizobakterienisolate. (Isolated Rhizobacteria for Treatment of Phytopathogenic Fungal Diseases). Patent EP 98124694.5, 31 March 1999. [Google Scholar]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019. [Google Scholar] [CrossRef]
- Elsas, J.D.v.; Chiurazzi, M.; Mallon, C.A.; Elhottovā, D.; Krištůfek, V.; Salles, J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. USA 2012, 109, 1159–1164. [Google Scholar] [CrossRef] [Green Version]
- Enebe, M.C.; Babalola, O.O. The impact of microbes in the orchestration of plants’ resistance to biotic stress: A disease management approach. Appl. Microbiol. Biotechnol. 2019, 103, 9–25. [Google Scholar] [CrossRef] [Green Version]
- De Vrieze, M.; Germanier, F.; Vuille, N.; Weisskopf, L. Combining different potato-associated Pseudomonas strains for improved biocontrol of Phytophthora infestans. Front. Microbiol. 2018, 9, 2573. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.D.; Kinkel, L.L.; Schottel, J.L. Effect of pathogen isolate, potato cultivar, and antagonist strain on potato scab severity and biological control. Biocontrol Sci. Technol. 2004, 14, 301–311. [Google Scholar] [CrossRef]
- Xue, A.G.; Chen, Y.; Voldeng, H.D.; Fedak, G.; Savard, M.E.; Längle, T.; Zhang, J.; Harman, G.E. Concentration and cultivar effects on efficacy of CLO-1 biofungicide in controlling Fusarium head blight of wheat. Biol. Control 2014, 73, 2–7. [Google Scholar] [CrossRef]
- Glaeser, S.P.; Gabur, I.; Haghighi, H.; Bartz, J.-O.; Kämpfer, P.; Snowdon, R.; Obermeier, C. Endophytic bacterial communities of oilseed rape associate with genotype-specific resistance against Verticillium longisporum. FEMS Microbiol. Ecol. 2020, 96, fiz188. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Germaine, K.J.; Forristal, P.D.; Spink, J.; Dowling, D.N. Meta-omics approach to unravel the endophytic bacterial communities of Brassica napus and other agronomically important crops in response to agricultural practices. In Endophytes for a Growing World; Cambridge University Press: Cambridge, UK, 2019; pp. 232–249. [Google Scholar]
- Abuamsha, R.; Salman, M.; Ehlers, R.-U. Differential resistance of oilseed rape cultivars (Brassica napus ssp. oleifera) to Verticillium longisporum infection is affected by rhizosphere colonisation with antagonistic bacteria, Serratia plymuthica and Pseudomonas chlororaphis. BioControl 2011, 56, 101–112. [Google Scholar] [CrossRef]
- Berg, G.; Raaijmakers, J.M. Saving seed microbiomes. ISME J. 2018, 12, 1167–1170. [Google Scholar] [CrossRef]
- Mallon, C.A.; Poly, F.; Le Roux, X.; Marring, I.; van Elsas, J.D.; Salles, J.F. Resource pulses can alleviate the biodiversity–invasion relationship in soil microbial communities. Ecology 2015, 96, 915–926. [Google Scholar] [CrossRef] [Green Version]
- Barret, M.; Guimbaud, J.; Darrasse, A.; Jacques, M. Plant microbiota affects seed transmission of phytopathogenic microorganisms. Mol. Plant Pathol. 2016, 17, 791. [Google Scholar] [CrossRef] [Green Version]
- Shtienberg, D.; Elad, Y. Incorporation of weather forecasting in integrated, biological-chemical management of Botrytis cinerea. Phytopathology 1997, 87, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Guetsky, R.; Shtienberg, D.; Elad, Y.; Fischer, E.; Dinoor, A. Improving biological control by combining biocontrol agents each with several mechanisms of disease suppression. Phytopathology 2002, 92, 976–985. [Google Scholar] [CrossRef] [Green Version]
- Bardin, M.; Ajouz, S.; Comby, M.; Lopez-Ferber, M.; Graillot, B.; Siegwart, M.; Nicot, P.C. Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Barret, M.; Morrissey, J.P.; O’Gara, F. Functional genomics analysis of plant growth-promoting rhizobacterial traits involved in rhizosphere competence. Biol. Fertil. Soils 2011, 47, 729. [Google Scholar] [CrossRef]
- Postma-Blaauw, M.B.; de Goede, R.G.M.; Bloem, J.; Faber, J.H.; Brussaard, L. Soil biota community structure and abundance under agricultural intensification and extensification. Ecology 2010, 91, 460–473. [Google Scholar] [CrossRef]
- Arima, K.; Imanaka, H.; Kousaka, M.; Fukuta, A.; Tamura, G. Pyrrolnitrin, a new antibiotic substance, produced by Pseudomonas. Agric. Biol. Chem. 1964, 28, 575–576. [Google Scholar] [CrossRef]
- Frankowski, J.; Lorito, M.; Scala, F.; Schmid, R.; Berg, G.; Bahl, H. Purification and properties of two chitinolytic enzymes of Serratia plymuthica HRO-C48. Arch. Microbiol. 2001, 176, 421–426. [Google Scholar] [CrossRef]
- Kaur, J.; Pethani, B.P.; Kumar, S.; Kim, M.; Sunna, A.; Kautto, L.; Penesyan, A.; Paulsen, I.T.; Nevalainen, H. Pseudomonas aeruginosa inhibits the growth of Scedosporium aurantiacum, an opportunistic fungal pathogen isolated from the lungs of cystic fibrosis patients. Front. Microbiol. 2015, 6, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashan, Y. Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol. Adv. 1998, 16, 729–770. [Google Scholar] [CrossRef]
- SPIonWeb. Available online: http://spionweb.tugraz.at/en/spi (accessed on 23 April 2020).
- Inderbitzin, P.; Ward, J.; Barbella, A.; Solares, N.; Izyumin, D.; Burman, P.; Chellemi, D.O.; Subbarao, K.V. Soil microbiomes associated with verticillium wilt-suppressive broccoli and chitin amendments are enriched with potential biocontrol agents. Phytopathology 2018, 108, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Köberl, M.; Ramadan, E.M.; Adam, M.; Cardinale, M.; Hallmann, J.; Heuer, H.; Smalla, K.; Berg, G. Bacillus and Streptomyces were selected as broad-spectrum antagonists against soilborne pathogens from arid areas in Egypt. FEMS Microbiol. Lett. 2013, 342, 168–178. [Google Scholar] [CrossRef] [Green Version]
- Messner, R.; Schweigrofler, W.; Ibl, M.; Berg, G.; Prillinger, H. Molecular characterization of the plant pathogen Verticillium dahliae Kleb. using RAPD-PCR and sequencing of the 18SrRNA-gene. J. Phytopathol. 1996, 144, 347–354. [Google Scholar] [CrossRef]
- Abuamsha, R.; Salman, M.; Ehlers, R.-U. Effect of seed priming with Serratia plymuthica and Pseudomonas chlororaphis to control Leptosphaeria maculans in different oilseed rape cultivars. Eur. J. Plant Pathol. 2011, 130, 287–295. [Google Scholar] [CrossRef]
- Narodoslawsky, M.; Stoeglehner, G. Planning for local and regional energy strategies with the ecological footprint. J. Environ. Policy Plan. 2010, 12, 363–379. [Google Scholar] [CrossRef]
- Čuček, L.; Klemeš, J.J.; Kravanja, Z. A review of footprint analysis tools for monitoring impacts on sustainability. J. Clean. Prod. 2012, 34, 9–20. [Google Scholar] [CrossRef]
- ISO. 14040: 2006 Environmental Management-Life Cycle Assessment-Principles and Framework; European Committee for Standardization: Brussels, Belgium, 2006. [Google Scholar]
- Kollmann, R.; Friebel, F.; Neugebauer, G.; Narodoslawsky, M. Process evaluation of the ecological performance of food and energy plant production systems. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 2187–2192. ISBN 1570-7946. [Google Scholar]
- Shahzad, K.; Kollmann, R.; Maier, S.; Narodoslawsky, M. SPIonWEB – Ecological Process Evaluation with the Sustainable Process Index (SPI). In Computer Aided Chemical Engineering; Klemeš, J.J., Varbanov, P.S., Liew, P.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 33, pp. 487–492. [Google Scholar]




| Parameter | Singular Bacterial Treatment | Dual Treatment | |||||
|---|---|---|---|---|---|---|---|
| Treatment/Parameter | S. plymuthica HRO-C48 | P. polymyxa Sb3-1 | S. plymuthica F20 | P. protegens F37 | P. fluorescens F2 | F37 + HRO-C48 | F2 + HRO-C48 |
| Fresh weight of 10 plants [mg] (1) | 464 ± 38 (409 ± 9.1) | 260 ± 30 * (409 ± 9.1) | 509 ± 86 (337 ± 72) | 370 ± 26 (337 ± 72) | 758 ± 187 *(337 ± 72) | 627 ± 45(591 ± 34) | 356 ± 58 (591 ± 34) |
| Germination (%) | 96 (100) | 75 * (100) | 86 (75) | 66 * (75) | 73 (75) | 95 (93) | 82 (93) |
| Reference | [44] | [44] | [42] | [42] | [42] | This study | This study |
| Field Trial 2016–2017 (Winter) | Number of Plants per m2 | % Verticillium-Infected Plants | Yield (kg/ha) | |||
|---|---|---|---|---|---|---|
| Strain/oilseed rape cultivar | Lockarp | Stångby | Lockarp | Stångby | Lockarp | Stångby |
| Untreated/ Avatar | 29.3 ± 2.9 | 37.3 ± 5.2 | 42.4 ± 4.1 | 50.3 ± 4.5 | 4173.7 ± 162.6 | 4663.2 ± 108.8 |
| F37/Avatar | 31.8 ± 2.6 | 30.8 ± 0.9 | 48.7 ± 5.3 | 40.8 ± 8.8 | 4461.3 ± 101.1 | 4637.0 ± 114.3 |
| Untreated/ Sherpa | 34.3 ± 2.8 | 34.3 ± 3.6 | 40.6 ± 3.2 | 46.0 ± 4.6 | 4090.2 ± 118.6 | 4700.4 ± 93.9 |
| F37/Sherpa | 8.8 ± 2.1 * | 33.5 ± 4.9 | 34.0 ± 3.2 | 53.2 ± 11.2 | 2529.7 ± 164.1 * | 4412.2 ± 276.0 |
| Field trial 2017 (summer) | Number of plants per m2 | % Verticillium-infected plants | Yield (kg/ha) | |||
| Sellersberga | Kärrarp | Sellersberga | Kärrarp | |||
| Untreated/ Avatar | 63.5 ± 4.6 | 29.8 ± 3.2 | 25.0 ± 2.9 | 17.5 ± 4.8 | n/a | |
| F20/Avatar | 51.8 ± 9.7 | 22.8 ± 1.4 | 12.5 ± 4.8 * | 10.0 ± 4.1 | n/a | |
| HRO-C48/Avatar | 52.8 ± 6.2 | 18.3 ± 3.4* | 20.0 ± 4.1 | 15.0 ± 2.9 | n/a | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybakova, D.; Wikström, M.; Birch-Jensen, F.; Postma, J.; Ehlers, R.U.; Schmuck, M.; Kollmann, R.; Köhl, J.; Berg, G. Verticillium Wilt in Oilseed Rape—the Microbiome is Crucial for Disease Outbreaks as Well as for Efficient Suppression. Plants 2020, 9, 866. https://doi.org/10.3390/plants9070866
Rybakova D, Wikström M, Birch-Jensen F, Postma J, Ehlers RU, Schmuck M, Kollmann R, Köhl J, Berg G. Verticillium Wilt in Oilseed Rape—the Microbiome is Crucial for Disease Outbreaks as Well as for Efficient Suppression. Plants. 2020; 9(7):866. https://doi.org/10.3390/plants9070866
Chicago/Turabian StyleRybakova, Daria, Mariann Wikström, Fia Birch-Jensen, Joeke Postma, Ralf Udo Ehlers, Maria Schmuck, René Kollmann, Jürgen Köhl, and Gabriele Berg. 2020. "Verticillium Wilt in Oilseed Rape—the Microbiome is Crucial for Disease Outbreaks as Well as for Efficient Suppression" Plants 9, no. 7: 866. https://doi.org/10.3390/plants9070866