Thermal Requirements Underpinning Germination Allude to Risk of Species Decline from Climate Warming
Abstract
:1. Introduction
2. Results
3. Discussion
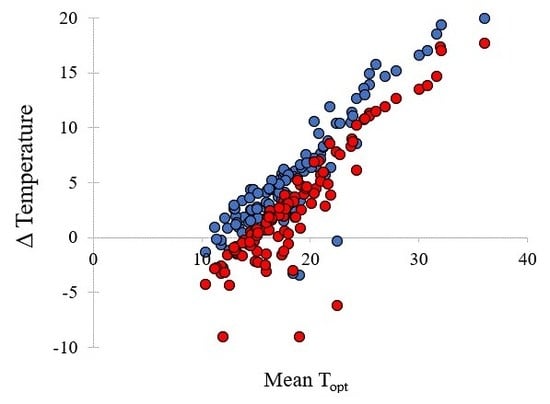
3.1. Species Vulnerability
3.2. Temperature Preferences
3.3. Restricted Versus Common Species Response
3.4. Limitations
4. Methods
4.1. Species, Climate and Habitat
4.2. Experimental Design
4.3. Data Analysis
4.4. Ethical Statement
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, U.; Breman, E.; Cossu, T.A.; Kenney, S. The conservation value of germplasm stored at the Millennium Seed Bank, Royal Botanic Gardens, Kew, UK. Biodivers. Conserv. 2018, 27, 1347–1386. [Google Scholar] [CrossRef] [Green Version]
- Cochrane, A. Western Australia’s ex situ conservation program for threatened species: A model integrated strategy for conservation. In Ex Situ Conservation: Supporting Species Survival in the Wild; Guerrant, E.O., Jr., Havens, K., Maunder, M., Eds.; Island Press: Washington, DC, USA, 2004. [Google Scholar]
- Cochrane, J.A.; Crawford, A.D.; Monks, L.T. The significance of ex situ seed conservation to reintroduction of threatened plants. Aust. J. Bot. 2007, 55, 356–361. [Google Scholar] [CrossRef]
- Abeli, T.; Dalrymple, S.; Godefroid, S.; Mondoni, A.; Müller, J.V.; Rossi, G.; Orsenigo, S. Ex situ collections and their potential for the restoration of extinct plants. Conserv. Biol. 2020, 34, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, A.; Kelly, A.; Brown, K.; Cunneen, S. Relationships between seed germination requirements and ecophysiological characteristics aid the recovery of threatened native plant species in Western Australia. Ecol. Manag. Restor. 2002, 3, 45–58. [Google Scholar] [CrossRef]
- Godefroid, S.; Van de Vyver, A.; Vanderborght, T. Germination capacity and viability of threatened species collections in seed banks. Biodivers. Conserv. 2010, 19, 1365–1383. [Google Scholar] [CrossRef]
- Shearer, B.L.; Crane, C.E.; Barrett, S.; Cochrane, A. Phytophthora cinnamomi invasion, a major threatening process to conservation of flora diversity in the South-west Botanical Province of Western Australia. Aust. J. Bot. 2007, 55, 225–238. [Google Scholar] [CrossRef]
- Cochrane, A. Can sensitivity to temperature during germination help predict global warming vulnerability? Seed Sci. Res. 2016, 26, 14–29. [Google Scholar] [CrossRef] [Green Version]
- Cochrane, A. Salt and waterlogging stress impacts on seed germination and early seedling growth of selected endemic plant species from Western Australia. Plant Ecol. 2018, 219, 633–647. [Google Scholar] [CrossRef]
- Cochrane, A.; Daws, M.I.; Hay, F.R. Seed-based approach for identifying flora at risk from climate warming. Austral. Ecol. 2011, 36, 923–935. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Chang. Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Probert, R.J. The role of temperature in the regulation of seed dormancy and germination. In Seeds: The Ecology of Regeneration in Plant Communities, 2nd ed.; Fenner, M., Ed.; CABI International: Wallingford, UK, 2000; pp. 261–292. [Google Scholar]
- Finch-Savage, W.E.; Phelps, K. Onion (Allium cepa) seedling emergence patterns can be explained by the influence of soil temperature and water potential on seed germination. J. Exp. Bot. 1993, 44, 407–414. [Google Scholar] [CrossRef]
- Roberts, E.H. Temperature and seed germination. Symp. Soc. Exp. Biol. 1988, 42, 109–132. [Google Scholar] [PubMed]
- Saatkamp, A.; Cochrane, A.; Commander, L.; Guja, L.K.; Jimenez-Alfaro, B.; Larson, J.; Nicotra, A.; Poschlod, P.; Silveira, F.A.O.; Cross Adam, T.; et al. A research agenda for seed-trait functional ecology. N. Phytol. 2019, 221, 1764–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donohue, K. Seeds and seasons: Interpreting germination timing in the field. Seed Sci. Res. 2005, 15, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Donohue, K.; Rubio de Casas, R.; Burghardt, L.; Kovach, K.; Willis, C. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Simons, A.M. Modes of response to environmental change and the elusive empirical evidence for bet hedging. Proc. R. Soc. B Biol. Sci. 2011, 278, 1601–1609. [Google Scholar] [CrossRef] [Green Version]
- Donohue, K. Germination timing influences natural selection on life-history characters in Arabidopsis thaliana. Ecology 2002, 83, 1006–1016. [Google Scholar]
- Rosbakh, S.; Poschlod, P. Initial temperature of seed germination as related to species occurrence along a temperature gradient. Funct. Ecol. 2015, 29, 5–14. [Google Scholar] [CrossRef]
- Larson, J.E.; Funk, J.L. Regeneration: An overlooked aspect of trait-based plant community assembly models. J. Ecol. 2016, 104, 1284–1298. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Alfaro, B.; Silveira, F.A.O.; Fidelis, A.; Poschlod, P.; Commander, L.E. Seed germination traits can contribute better to plant community ecology. J. Veg. Sci. 2016, 23, 637–645. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977; p. 892. [Google Scholar]
- Gioria, M.; Pyšek, P.; Osborne, B.A. Timing is everything: Does early and late germination favor invasions by herbaceous alien plants? J. Plant Ecol. 2018, 11, 4–16. [Google Scholar] [CrossRef]
- Leverett, L.D.; Schieder IV, G.F.; Donohue, K. The fitness benefits of germinating later than neighbors. Am. J. Bot. 2018, 105, 20–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moles, A.T.; Westoby, M. What do seedlings die from and what are the implications for evolution of seed size? Oikos 2004, 106, 193–199. [Google Scholar] [CrossRef]
- Mondoni, A.; Rossi, G.; Orsenigo, S.; Probert, R.J. Climate warming could shift the timing of seed germination in alpine plants. Ann. Bot. 2012, 110, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Ooi, M.K.J. Delayed emergence and post-fire recruitment success: Effects of seasonal germination, fire season and dormancy type. Aust. J. Bot. 2010, 58, 248–256. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014; p. 1586. [Google Scholar]
- IPPC. Climate Change 2013: The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Daibes, L.F.; Amoêdo, S.C.; do Nascimento Moraes, J.; Fenelon, N.; da Silva, D.R.; de Melo Lopes, M., Jr.; Vargas, L.A.; Monteiro, E.F.; Frigeri, R.B.C. Thermal requirements of seed germination of ten tree species occurring in the western Brazilian Amazon. Seed Sci. Res. 2019, 29, 115–123. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Zhou, D. A modified thermal time model quantifying germination response to temperature for C3 and C4 species in temperate grassland. Agriculture 2015, 5, 412–426. [Google Scholar] [CrossRef] [Green Version]
- Seal, C.E.; Daws, M.I.; Flores, J.; Ortega-Baes, P.; Galíndez, G.; León-Lobos, P.; Sandoval, A.; Ceroni Stuva, A.; Ramírez Bullón, N.; Dávila-Aranda, P.; et al. Thermal buffering capacity of the germination phenotype across the environmental envelope of the Cactaceae. Glob. Chang. Biol. 2017, 23, 5309–5317. [Google Scholar] [CrossRef]
- Qiu, J.; Bai, Y.; Coulman, B.; Romo, J.T. Using thermal time models to predict seedling emergence of orchardgrass (Dactylis glomerata L.) under alternating temperature regimes. Seed Sci. Res. 2006, 16, 261–271. [Google Scholar] [CrossRef]
- Galíndez, G.; Seal, C.E.; Daws, M.I.; Lindow, L.; Ortega-Baes, P.; Pritchard, H.W. Alternating temperature combined with darkness resets base temperature for germination (Tb) in photoblastic seeds of Lippia and Aloysia (Verbenaceae). Plant Biol. 2016, 19, 41–45. [Google Scholar] [CrossRef]
- Moot, D.J.; Scott, W.R.; Roy, A.M.; Nicholls, A.C. Base temperature and thermal time requirements for germination and emergence of temperate pasture species. N. Z. J. Agric. Res. 2000, 43, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, J.M.; Erickson, T.E. Warmer seed environments increase germination fractions in Australian winter annual plant species. Ecosphere 2016, 7, e01497. [Google Scholar] [CrossRef]
- Hoyle, G.L.; Venn, S.E.; Steadman, K.J.; Good, R.B.; McAuliffe, E.J.; Williams, E.R.; Nicotra, A.B. Soil warming increases plant species richness but decreases germination from the alpine soil seed bank. Glob. Chang. Biol. 2013, 19, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, A.; Hoyle, G.L.; Yates, C.J.; Wood, J.; Nicotra, A.B. Climate warming delays and decreases seedling emergence in a Mediterranean ecosystem. Oikos 2015, 124, 150–160. [Google Scholar] [CrossRef]
- Briceño, V.; Hoyle, G.; Nicotra, A. Seeds at risk: How will a changing alpine climate affect regeneration from seeds in alpine areas? Alp. Bot. 2015, 125, 59–68. [Google Scholar] [CrossRef]
- Bush, A.; Catullo, R.A.; Mokany, K.; Thornhill, A.H.; Miller, J.T.; Ferrier, S. Truncation of thermal tolerance niches among Australian plants. Glob. Ecol. Biogeogr. 2018, 27, 22–31. [Google Scholar] [CrossRef]
- Booth, T.H. Assessing species climatic requirements beyond the realized niche: Some lessons mainly from tree species distribution modelling. Clim. Chang. 2017, 145, 259–271. [Google Scholar] [CrossRef]
- Klausmeyer, K.R.; Shaw, M.R. Climate change, habitat loss, protected areas and the climate adaptation potential of species in Mediterranean ecosystems worldwide. PLoS ONE 2009, 4, e6392. [Google Scholar] [CrossRef] [Green Version]
- Steffen, W.; Hughes, L. The Critical Decade: Western Australia Climate Change Impacts; Climate Commission Secretariat: Canberra, ACT, Australia, 2011.
- Fitzpatrick, M.C.; Gove, A.D.; Sanders, N.J.; Dunn, R.R. Climate change, plant migration, and range collapse in a global biodiversity hotspot: The Banksia (Proteaceae) of Western Australia. Glob. Chang. Biol. 2008, 14, 1337–1352. [Google Scholar] [CrossRef]
- Cochrane, A. Modelling seed germination response to temperature in Eucalyptus L’Her. (Myrtaceae) species in the context of global warming. Seed Sci. Res. 2017, 27, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Cochrane, A. Temperature thresholds for germination in 20 short-range endemic plant species from a Greenstone Belt in southern Western Australia. Plant Biol. 2020, 22, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, A.; Hoyle, G.L.; Yates, C.J.; Wood, J.; Nicotra, A.B. Predicting the impact of increasing temperatures on seed germination among populations of Western Australian Banksia (Proteaceae). Seed Sci. Res. 2014, 24, 195–205. [Google Scholar] [CrossRef]
- Bell, D.T.; Plummer, J.A.; Taylor, S.K. Seed germination ecology in southwestern Western Australia. Bot. Rev. 1993, 59, 25–73. [Google Scholar] [CrossRef]
- Santana, V.M.; Baeza, M.J.; Blanes, M.C. Clarifying the role of fire heat and daily temperature fluctuations as germination cues for Mediterranean Basin obligate seeders. Ann. Bot. 2013, 111, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, S.T.; Betancourt, J.L.; Booth, R.K.; Gray, S.T. Ecology and the ratchet of events: Climate variability, niche dimensions, and species distributions. Proc. Natl. Acad. Sci. USA 2009, 106, 19685–19692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, O.S.; Heskel, M.A.; Reich, P.B.; Tjoelker, M.G.; Weerasinghe, L.K.; Penillard, A.; Zhu, L.; Egerton, J.J.G.; Bloomfield, K.J.; Creek, D.; et al. Thermal limits of leaf metabolism across biomes. Glob. Chang. Biol. 2017, 23, 209–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, L.; Cawsey, E.M.; Westoby, M. Climatic range sizes of Eucalyptus species in relation to future climate change. Glob. Ecol. Biogeogr. Lett. 1996, 5, 23–29. [Google Scholar] [CrossRef]
- Hope, P.; Abbs, D.; Bhend, J.; Chiew, F.; Church, J.; Ekström, M.; Kirono, D.; Lenton, A.; Lucas, C.; McInnes, K.; et al. Southern and South-Western Flatlands cluster report. In Climate Change in Australia Projections for Australia’s Natural Resource Management Regions; Ekström, M., Whetton, P., Gerbing, C., Grose, M., Webb, L., Risbey, J., Eds.; CSIRO and Bureau of Meteorology: Canberra, ACT, Australia, 2015; pp. 1–58. [Google Scholar]
- Liu, K.; Baskin, J.M.; Baskin, C.C.; Bu, H.; Du, G.; Ma, M. Effect of Diurnal Fluctuating versus Constant Temperatures on Germination of 445 Species from the Eastern Tibet Plateau. PLoS ONE 2013, 8, e69364. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Pascual, E.; Jiménez-Alfaro, B.; Díaz, T. The temperature dimension of the seed germination niche in fen wetlands. Plant Ecol. 2013, 214, 489–499. [Google Scholar] [CrossRef]
- Thompson, K.; Grime, J.P.; Mason, G. Seed germination in response to diurnal fluctuations of temperature. Nature 1977, 267, 147–149. [Google Scholar] [CrossRef]
- Bevill, R.L.; Louda, S.M. Comparisons of related rare and common species in the study of plant rarity. Conserv. Biol. 1999, 13, 493–498. [Google Scholar] [CrossRef]
- Murray, B.R.; Thrall, P.H.; Gill, A.M.; Nicotra, A.B. How plant life-history and ecological traits relate to species rarity and commonness at varying spatial scales. Austral. Ecol. 2002, 27, 291–310. [Google Scholar] [CrossRef]
- Brown, J.; Enright, N.J.; Miller, B.P. Seed production and germination in two rare and three common co-occurring Acacia species from south-east Australia. Austral. Ecol. 2003, 28, 271–280. [Google Scholar] [CrossRef]
- Paulů, A.; Harčariková, L.; Münzbergová, Z. Are there systematic differences in germination between rare and common species? A case study from central European mountains. Flora 2017, 236–237, 15–24. [Google Scholar] [CrossRef]
- Cochrane, A. Multi-year sampling provides insight into the bet-hedging capacity of the soil-stored seed reserve of a threatened Acacia species from Western Australia. Plant Ecol. 2019, 220, 241–253. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Cochrane, A.; Yates, C.J.; Hoyle, G.L.; Nicotra, A.B. Will among-population variation in seed traits improve the chance of species persistence under climate change? Glob. Ecol. Biogeogr. 2015, 24, 12–24. [Google Scholar] [CrossRef]
- Gray, F.; Cochrane, A.; Poot, P. Provenance modulates sensitivity of stored seeds of the Western Australian native grass Neurachne alopecuroidea to temperature and moisture availability. Aust. J. Bot. 2019, 67, 106–115. [Google Scholar] [CrossRef]
- Beard, J.S. Plant Life of Western Australia, 2nd ed.; Rosenberg Publishing: Sydney, Australia, 2015; p. 325. [Google Scholar]
- Hopper, S.D.; Gioia, P. The SouthWest Australian Floristic Region: Evolution and conservation of a global hot spot of biodiversity. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 623–650. [Google Scholar] [CrossRef]
- Liyanage, G.S.; Ooi, M.K.J. Do dormancy-breaking temperature thresholds change as seeds age in the soil seed bank? Seed Sci. Res. 2017, 27, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cuena-Lombraña, A.; Sanna, M.; Porceddu, M.; Bacchetta, G. Does Storage under Gene Bank Conditions Affect Seed Germination and Seedling Growth? The Case of Senecio morisii (Asteraceae), a Vascular Plant Exclusive to Sardinian Water Meadows. Plants 2020, 9, 581. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.C.; Baskin, J.M. Breaking Seed Dormancy during Dry Storage: A Useful Tool or Major Problem for Successful Restoration via Direct Seeding? Plants 2020, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Ooi, M.K.J. Seed bank persistence and climate change. Seed Sci. Res. 2012, 22, S53–S60. [Google Scholar] [CrossRef] [Green Version]
- Cochrane, A. Are we underestimating the impact of rising summer temperatures on dormancy loss in hard-seeded species? Aust. J. Bot. 2017, 65, 248–256. [Google Scholar] [CrossRef]
- Fernández-Pascual, E.; Mattana, E.; Pritchard, H.W. Seeds of future past: Climate change and the thermal memory of plant reproductive traits. Biol. Rev. 2019, 94, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Jones, A. Department of Biodiversity, Conservation and Attractions Threatened and Priority Flora List. Available online: https://www.dpaw.wa.gov.au/plants-and-animals/threatened-species-and-communities/threatened-plants (accessed on 16 January 2018).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Moss, R.H.; Edmonds, J.A.; Hibbard, K.A.; Manning, M.R.; Rose, S.K.; van Vuuren, D.P.; Carter, T.R.; Emori, S.; Kainuma, M.; Kram, T.; et al. The next generation of scenarios for climate change research and assessment. Nature 2010, 463, 747–756. [Google Scholar] [CrossRef]
- Arnold, S.M.; Monteith, J.L. Plant development and mean temperature in a Teesdale habitat. J. Ecol. 1974, 62, 711–720. [Google Scholar] [CrossRef]
- Trudgill, D.L.; Honek, A.; Li, D.; Van Straalen, N.M. Thermal time—Concepts and utility. Ann. Appl. Biol. 2005, 146, 1–14. [Google Scholar] [CrossRef]








| Mean | Min | Max | Median | SD | ||
|---|---|---|---|---|---|---|
| Germination (%) | C | 95.4 | 8.0 | 100.0 | 100.0 | 14.41 |
| R | 98.4 | 58.0 | 100.0 | 100.0 | 7.46 | |
| T0 (lag time) (days) | C | 11.9 | 2.0 | 46.0 | 11.0 | 7.93 |
| R | 11.7 | 3.0 | 46.0 | 11.0 | 7.25 | |
| T50 (days) | C | 14.3 | 3.0 | 53.0 | 13.0 | 8.69 |
| R | 14.8 | 7.0 | 62.0 | 13.0 | 9.84 | |
| diff T0 to T50 (days) | C | 3.2 | 0 | 21 | 2 | 4.17 |
| R | 3.1 | 0 | 16 | 3 | 3.03 | |
| 1/T50 | C | 0.098 | 0.019 | 0.333 | 0.0769 | 0.069 |
| R | 0.083 | 0.016 | 0.143 | 0.0769 | 0.032 | |
| MTG (days) | C | 15.5 | 3.0 | 55.6 | 13.8 | 9.49 |
| R | 15.6 | 7.0 | 54.7 | 14.2 | 8.67 | |
| Day temperature (°C) | C | 22.2 | 12.8 | 41.5 | 20.9 | 6.19 |
| R | 21.1 | 12.0 | 36.4 | 22.1 | 5.06 | |
| Night temperature (°C) | C | 15.4 | 5.4 | 31.2 | 15.0 | 5.58 |
| R | 14.9 | 5.1 | 25.0 | 14.8 | 5.59 | |
| Amplitude (°C) | C | 6.8 | 0.4 | 22.6 | 5.9 | 4.87 |
| R | 6.3 | 0.0 | 28.1 | 4.0 | 6.32 | |
| Mean Topt (°C) | C | 18.8 | 10.4 | 36.1 | 17.6 | 5.34 |
| R | 17.7 | 11.8 | 25.5 | 17.2 | 4.06 | |
| Degree days for T50% | C | 255 | 75 | 1288 | 225 | 169 |
| R | 247 | 105 | 924 | 226 | 147 |
| Species | Day Temperature | Night Temperature | Δ Temperature | Mean Temperature | |||||
|---|---|---|---|---|---|---|---|---|---|
| Regression Coefficient | R2 | Regression Coefficient | R2 | Regression Coefficient | R2 | Regression Coefficient | R2 | ||
| lag | all | −0.5950 * | 0.047 | −1.308 *** | 0.254 | 0.115 * | 0.053 | −1.298 *** | 0.153 |
| common | −0.3670 * | 0.065 | −1.067 *** | 0.198 | 0.0110 | 0.000 | −1.286 *** | 0.156 | |
| restricted | −2.4100 | 0.002 | −1.797 *** | 0.378 | 0.5060 * | 0.182 | −1.310 | 0.093 | |
| 1/T50 | all | 0.0003 *** | 0.251 | 0.0078 ** | 0.423 | 0.0038 | 0.009 | 0.0080 *** | 0.404 |
| common | 0.0015 *** | 0.281 | 0.0108 *** | 0.417 | 0.0061 | 0.000 | 0.0082 *** | 0.399 | |
| restricted | 0.0173 * | 0.252 | 0.0002 *** | 0.602 | −0.0012 * | 0.123 | 0.0055 *** | 0.331 | |
| MTG | all | −0.683 * | 0.045 | −1.2060 *** | 0.229 | 0.1250 * | 0.045 | −1.282 *** | 0.144 |
| common | −0.300 * | 0.055 | −0.7450 *** | 0.169 | 0.0330 | 0.000 | −1.000 ** | 0.130 | |
| restricted | −2.910 | 0.034 | −2.1800 *** | 0.389 | 0.0547 ** | 0.154 | −2.340 ** | 0.137 | |
| Species | Family | Cons.code | Endemic | Obligate Seeder | Provenance | IBRA |
|---|---|---|---|---|---|---|
| Acacia besleyi * | Fabaceae | P1 | Y | Y | Ravensthorpe | ESP |
| Acacia bifaria * | Fabaceae | P3 | Y | Y | Ravensthorpe | ESP |
| Acacia disticha * | Fabaceae | - | Y | Y | Ravensthorpe | ESP |
| Acacia durabilis * | Fabaceae | - | Y | Y | Ravensthorpe | ESP |
| Acacia heterochroa * | Fabaceae | - | Y | Y | Ravensthorpe | ESP |
| Acacia ophiolithica * | Fabaceae | - | Y | Y | Ravensthorpe | ESP |
| Acacia pinguiculosa * | Fabaceae | - | Y | Y | Ravensthorpe | ESP |
| Allocasuarina hystricosa | Allocasuarinaceae | P4 | Y | N | Ravensthorpe | ESP |
| Anigozanthus manglesii subsp. Quadrans # | Haemodoraceae | - | Y | Y | Enneaba | GES |
| Banksia aculeata | Proteaceae | P2 | Y | Y | Stirling Range NP | ESP |
| Banksia ashbyi | Proteaceae | - | Y | Y | Ajana | GES |
| Banksia attenuata | Proteaceae | Y | N | Brighton | SWA | |
| Banksia attenuata | Proteaceae | - | Y | N | Carnamah-Eneabba | GES |
| Banksia attenuata | Proteaceae | - | Y | N | Kalbarri NP | GES |
| Banksia baueri | Proteaceae | - | Y | Y | Stirling Range NP | ESP |
| Banksia baueri | Proteaceae | - | Y | Y | Fitzgerald River NP | ESP |
| Banksia baueri | Proteaceae | - | Y | Y | Tarin Rock NR | AVW |
| Banksia baxteri | Proteaceae | - | Y | Y | Stirling Range NP | ESP |
| Banksia benthamiana | Proteaceae | P4 | Y | Y | Nudagong East Rd | AVW |
| Banksia blechnifolia | Proteaceae | - | Y | Y | Fitzgerald | ESP |
| Banksia brownii | Proteaceae | DRF | Y | Y | Vancouver Peninsula | ESP |
| Banksia burdettii | Proteaceae | - | Y | Y | Agaton Rd Reserve | GES |
| Banksia caleyi | Proteaceae | - | Y | Y | Stirling Range NP | ESP |
| Banksia coccinea | Proteaceae | - | Y | Y | Gull Rock NP | ESP |
| Banksia dryandroides | Proteaceae | - | Y | Y | Cheyne Rd NR | ESP |
| Banksia grandis | Proteaceae | - | Y | N | Marbellup | ESP |
| Banksia hookeriana | Proteaceae | - | Y | Y | Kalbarri NP | GES |
| Banksia laevigata subsp. laevigata | Proteaceae | P4 | Y | Y | Ravensthorpe Range | ESP |
| Banksia lanata | Proteaceae | - | Y | Y | Badgingarra | GES |
| Banksia laricina | Proteaceae | - | Y | Y | Moore River NP | GES |
| Banksia lemanniana | Proteaceae | - | Y | Y | Ravensthorpe Range | ESP |
| Banksia leptophylla var. mellitica | Proteaceae | - | Y | Y | Coorow-Greenhead | GES |
| Banksia lindleyana | Proteaceae | - | Y | Y | Binnu West | GES |
| Banksia media | Proteaceae | - | Y | Y | Cape Arid NP | ESP |
| Banksia meisneri subsp. ascendens | Proteaceae | P4 | Y | Y | Scott River | SWA |
| Banksia nutans | Proteaceae | - | Y | Y | Cape Arid NP | ESP |
| Banksia occidentalis | Proteaceae | - | Y | Y | Hay River | ESP |
| Banksia oreophila | Proteaceae | - | Y | Y | Hamilla Hills | ESP |
| Banksia petiolaris | Proteaceae | - | Y | Y | Jerdacuttup | ESP |
| Banksia pilostylis | Proteaceae | - | Y | Y | Springdale Rd | ESP |
| Banksia pilostylis | Proteaceae | - | Y | Y | Mt Howick | ESP |
| Banksia praemorsa | Proteaceae | - | Y | Y | Torndirrup NP | ESP |
| Banksia prionotes | Proteaceae | - | Y | Y | Carnamah-Eneabba | GES |
| Banksia prionotes | Proteaceae | - | Y | Y | Kalbarri NP | GES |
| Banksia pulchella | Proteaceae | - | Y | Y | Cape Arid NP | ESP |
| Banksia quercifolia | Proteaceae | - | Y | Y | Rudgyard NR | ESP |
| Banksia scabrella | Proteaceae | P4 | Y | Y | Burma Rd NR | GES |
| Banksia sceptrum | Proteaceae | - | Y | Y | Ajana | GES |
| Banksia seminuda | Proteaceae | - | Y | Y | Manjimup | SWA |
| Banksia solandri | Proteaceae | P2 | Y | Y | Stirling Range NP | ESP |
| Banksia speciosa | Proteaceae | - | Y | Y | Cape Arid NP | ESP |
| Banksia sphaerocarpa | Proteaceae | - | Y | N | Tarin Rock NR | AVW |
| Banksia telmateiae | Proteaceae | - | Y | Y | Jurien | GES |
| Banksia verticillata | Proteaceae | DRF | Y | Y | Torndirrup NP | ESP |
| Banksia victoriae | Proteaceae | - | Y | Y | Ajana | GES |
| Banksia violaceae | Proteaceae | - | Y | Y | Munglinup | ESP |
| Banksia violaceae | Proteaceae | - | Y | Y | Tarin Rock NR | ESP |
| Beaufortia orbifolia | Myrtaceae | Y | Y | Ravensthorpe | ESP | |
| Borya sphaerocephela | Boryaceae | Y | N | Perth Hills | SWA | |
| Callitris pyramidalis | Cupressaceae | Y | N | Camel Lake SRNP | ESP | |
| Calothamnus gracilis | Myrtaceae | Y | N | Chillinup | ESP | |
| Calothamnus roseus | Myrtaceae | P2 | Y | Y | Ravensthorpe | ESP |
| Carex tereticaulis | Cyperaceae | P1 | Y | Y | Harvey River | SWA |
| Daviesia megacalyx * | Fabaceae | DRF | Y | Y | Ravensthorpe | ESP |
| Eucalyptus aequiperta | Myrtaceae | Y | N | Narrogin | AVW | |
| Eucalyptus argutifolia | Myrtaceae | DRF | Y | N | Seabird | SWA |
| Eucalyptus burdettiana | Myrtaceae | DRF | Y | Y | FRNP | ESP |
| Eucalyptus calcicola subsp. unita | Myrtaceae | P4 | Y | N | West Cape Howe | ESP |
| Eucalyptus cernua | Myrtaceae | Y | Y | Ravensthorpe Range | ESP | |
| Eucalyptus conferruminata | Myrtaceae | Y | Y | Albany | ESP | |
| Eucalyptus crispata | Myrtaceae | DRF | Y | N | Bunney Rd | AVW |
| Eucalyptus desmondensis | Myrtaceae | P4 | Y | Y | Ravensthorpe Range | ESP |
| Eucalyptus erythrocorys | Myrtaceae | Y | N | Coorow-Greenhead | GES | |
| Eucalyptus georgei subsp. fulgida | Myrtaceae | P4 | Y | Y? | Forrestiana | MAL |
| Eucalyptus insularis | Myrtaceae | DRF | Y | Y | Cape le Grande | ESP |
| Eucalyptus jimberlanica | Myrtaceae | P1 | Y | N | Jimberlana Hill | COO |
| Eucalyptus kondininensis | Myrtaceae | Y | Y? | Narrogin | AVW | |
| Eucalyptus kruseana | Myrtaceae | P4 | Y | N | Cardunia Rocks | COO |
| Eucalyptus livida | Myrtaceae | Y | N | Ironcaps | MAL | |
| Eucalyptus marginata | Myrtaceae | Y | N | Wandi | SWA | |
| Eucalyptus megacarpa | Myrtaceae | Y | N | SRNP | ESP | |
| Eucalyptus megacarpa | Myrtaceae | Y | N | Torndirrup | ESP | |
| Eucalyptus megacornuta | Myrtaceae | Y | Y | Ravensthorpe | ESP | |
| Eucalyptus myriadena | Myrtaceae | Y | N | Lake Varley | MAL | |
| Eucalyptus newbeyi | Myrtaceae | P3 | Y | Y | Bremer Bay | ESP |
| Eucalyptus nigrifunda | Myrtaceae | P4 | Y | Y? | Mt Dennis | COO |
| Eucalyptus nutans | Myrtaceae | DRF | Y | Y | Bremer Bay | ESP |
| Eucalyptus pimpiniana | Myrtaceae | P3 | Y | N | Ponton Creek | COO |
| Eucalyptus preissiana subsp. lobata | Myrtaceae | P4 | Y | N | Starvation Boat Harbour | ESP |
| Eucalyptus rugulata | Myrtaceae | P4 | Y | Y | Ironcaps | MAL |
| Eucalyptus spathulata subsp. salina | Myrtaceae | P3 | Y | Y | Corrigin | AVW |
| Eucalyptus staeri | Myrtaceae | Y | N | Cheyne Beach | ESP | |
| Eucalyptus stoatei | Myrtaceae | P4 | Y | Y | Ravensthorpe Range | ESP |
| Eucalyptus subtilis | Myrtaceae | Y | N | Lake King | AVW | |
| Eucalyptus talyuberlup | Myrtaceae | Y | Y | SRNP | ESP | |
| Isopogon Ravensthorpe * | Proteaceae | Y | N | Ravensthorpe | ESP | |
| Kunzea acicularis ^ | Myrtaceae | DRF | Y | Y | Ravensthorpe | ESP |
| Leptospermum incanum ^ | Myrtaceae | Y | Y | Peak Charles | ESP | |
| Melaleuca penicula | Myrtaceae | Y | ? | Ravensthorpe | ESP | |
| Melaleuca preissiana | Myrtaceae | Y | Y | Jandakot | SWA | |
| Melaleuca stramentosa | Myrtaceae | Y | Y | Ravensthorpe Range | ESP | |
| Neurachne alopecuroidea | Poaceae | N | N | Tootbardie | AVW | |
| Neurachne alopecuroidea | Poaceae | N | N | Perth Hills | SWA | |
| Neurachne alopecuroidea | Poaceae | N | N | Narrogin | AVW | |
| Neurachne alopecuroidea | Poaceae | N | N | Cocanarup | ESP | |
| Podolepis aristata | Asteraceae | Y | Y | Cairn Hill | GES | |
| Regelia ciliata | Myrtaceae | Y | Y | Dobaderry Swamp | SWA | |
| Rhodanthe pyrethrum | Asteraceae | Y | Y | Brixton St | SWA | |
| Stylidium scandens | Stylidiaceae | - | Y | Y | Mt Lindesay | WAR |
| Xanthorrhoea preissii | Xanthorrhoeaceae | Y | N | Brookton | SWA | |
| Xerochrysum macranthum | Asteraceae | Y | Y | Porongurups | ESP | |
| Xyris lacera | Xyridaceae | Y | Y | Beardwood Rd | WAR | |
| Xyris maxima | Xyridaceae | P2 | Y | Y | Spearwood Swamp | WAR |
| Latitude | Longitude | Current MAP (mm) | Current MAT (°C) | MAP (2061–2080) | MAT (2061–2080) | Current Temperature in Wet Quarter (°C) | Temperature in Wet Quarter (2061–2080) (°C) | Seeds Per Gram | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | R | C | R | C | R | C | R | C | R | C | R | C | R | C | R | C | R | |
| Mean | 32.7 | 33.0 | 118.0 | 119.1 | 535.1 | 517.9 | 16.9 | 16.7 | 442.9 | 431.7 | 19.6 | 19.3 | 12.7 | 13.6 | 15.5 | 16.4 | 1912 | 1472 |
| Min | 27.6 | 29.1 | 114.3 | 115.2 | 301.0 | 238.0 | 13.8 | 14.3 | 272.0 | 233.0 | 16.5 | 16.6 | 9.9 | 10.2 | 13.3 | 14.0 | 6 | 9 |
| Max | 35.1 | 35.1 | 123.5 | 123.2 | 1126.0 | 1035.0 | 20.0 | 19.6 | 913.0 | 831.0 | 23.9 | 22.5 | 16.4 | 22.9 | 19.1 | 28.8 | 44,000 | 11,170 |
| Median | 33.6 | 33.6 | 118.0 | 119.8 | 460.0 | 415.0 | 16.3 | 16.2 | 384.0 | 346.0 | 19.0 | 18.7 | 12.4 | 12.7 | 15.0 | 15.3 | 76 | 633 |
| SD | 2.07 | 1.68 | 2.34 | 2.29 | 212.2 | 239.3 | 1.87 | 1.44 | 162.6 | 181.4 | 2.032 | 1.689 | 1.57 | 3.02 | 1.50 | 3.56 | 6007 | 2652 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cochrane, J.A. Thermal Requirements Underpinning Germination Allude to Risk of Species Decline from Climate Warming. Plants 2020, 9, 796. https://doi.org/10.3390/plants9060796
Cochrane JA. Thermal Requirements Underpinning Germination Allude to Risk of Species Decline from Climate Warming. Plants. 2020; 9(6):796. https://doi.org/10.3390/plants9060796
Chicago/Turabian StyleCochrane, Jennifer Anne. 2020. "Thermal Requirements Underpinning Germination Allude to Risk of Species Decline from Climate Warming" Plants 9, no. 6: 796. https://doi.org/10.3390/plants9060796