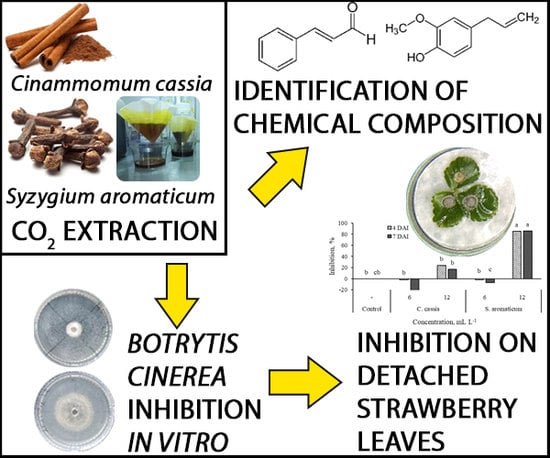
The Extracts of Cinnamon and Clove as Potential Biofungicides against Strawberry Grey Mould
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. The Extraction of Plant Material
4.2. Identification of the Extracts Chemical Composition
4.3. Antifungal Activity In Vitro
4.4. Antifungal Activity on Detached Strawberry Leaves
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mónaco, C.; Dal Bello, G.; Rollán, M.C.; Ronco, L.; Lampugnani, G.; Arteta, N.; Abramoff, C.; Aprea, A.; Larran, S.; Stocco, M. Biological control of Botrytis cinerea on tomato using naturally occurring fungal antagonists. Arch. Phytopathol. Plant Prot. 2009, 42, 729–737. [Google Scholar] [CrossRef]
- Daniel, C.K.; Lennox, C.L.; Vries, F.A. In vivo application of garlic extracts in combination with clove oil to prevent postharvest decay caused by Botrytis cinerea, Penicillium expansum and Neofabraea alba on apples. Postharvest Biol. Technol. 2015, 99, 88–92. [Google Scholar] [CrossRef]
- Rasiukevičiūtė, N.; Uselis, N.; Valiuškaitė, A. The use of forecasting model iMETOS® for strawberry grey mould management. Zemdirb. Agric. 2019, 106, 143–150. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P. Effects of resveratrol on the ultrastructure of Botrytis cinerea conidia and biological significance in plant/pathogen interactions. Fitoterapia 2012, 83, 1345–1350. [Google Scholar] [CrossRef]
- Elad, Y.; Vivier, M.; Fillinger, S. Botrytis, the good, the bad and the ugly. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 1–15. ISBN 9783319233710. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Adebayo, O.; Dang, T.; Bélanger, A.; Khanizadeh, S. Antifungal studies of selected essential oils and a commercial formulation against Botrytis cinerea. J. Food Res. 2013, 2, 217–226. [Google Scholar] [CrossRef]
- Absalan, A.; Mohiti-Ardakani, J.; Hadinedoushan, H.; Khalili, M.A. Hydro-alcoholic cinnamon extract, enhances glucose transporter isotype-4 translocation from intracellular compartments into the cytoplasmic membrane of C2C12 myotubes. Indian J. Clin. Biochem. 2012, 27, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid. Based Complement. Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef] [Green Version]
- Hajimonfarednejad, M.; Ostovar, M.; Raee, M.J.; Hashempur, M.H.; Mayer, J.G.; Heydari, M. Cinnamon: A systematic review of adverse events. Clin. Nutr. 2019, 38, 594–602. [Google Scholar] [CrossRef]
- Nurmalasari, D.L.; Damiyanti, M.; Eriwati, Y.K. Effect of cinnamon extract solution on human tooth enamel surface roughness. J. Phys. Conf. Ser. 2018, 1073. [Google Scholar] [CrossRef]
- Soliman, M.M.; Hamid, O.M.A.; Maqsood, H.A.; Aziza, S.; El Sanosi, Y.; Ragab, O.A. Insulin-mimetic effects of cinnamon extract in wistar rats. Lucr. Stiint. Ser. Med. Vet. 2015, 55, 61–68. [Google Scholar]
- Ahmed, J.; Altun, E.; Aydogdu, M.O.; Gunduz, O.; Kerai, L.; Ren, G.; Edirisinghe, M. Anti-fungal bandages containing cinnamon extract. Int. Wound J. 2019, 16, 730–736. [Google Scholar] [CrossRef] [Green Version]
- Cock, I.E.; Cheesman, M. Plants of the genus Syzygium (Myrtaceae): A review on ethnobotany, medicinal properties and phytochemistry. In Bioactive Compounds of Medicinal Plants: Properties and Potential for Human Health; Goyal, M.R., Ayeleso, A.O., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 35–84. ISBN 9781771886482. [Google Scholar]
- Hamini-Kadar, N.; Hamdane, F.; Boutoutaou, R.; Kihal, M.; Henni, J.E. Antifungal activity of clove (Syzygium aromaticum L.) essential oil against phytopathogenic fungi of tomato (Solanum lycopersicum L.) in Algeria. J. Exp. Biol. Agric. Sci. 2014, 2, 447–454. [Google Scholar]
- Eman-Abdeen, E.; El-Diasty, E.M. Antifungal activity of clove oil on dermatophytes and other fungi. Int. J. Adv. Res. 2015, 3, 1299–1305. [Google Scholar]
- Božik, M.; Nový, P.; Klouček, P. Chemical composition & antimicrobial activity of cinnamon, thyme, oregano & clove essential oils against plant pathogenic bacteria. Acta Univ. Agric. Silvic. Mendel. Brun. 2017, 65, 1129–1134. [Google Scholar] [CrossRef] [Green Version]
- Shabana, Y.M.; Abdalla, M.E.; Shahin, A.A.; El-Sawy, M.M.; Draz, I.S.; Youssif, A.W. Efficacy of plant extracts in controlling wheat leaf rust disease caused by Puccinia triticina. Egypt. J. Basic Appl. Sci. 2017, 4, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Šernaitė, L.; Valiuškaitė, A.; Rasiukevičiūtė, N.; Dambrauskienė, E.; Viškelis, P. The effect of spice extracts on strawberry pathogen Botrytis cinerea. In Proceedings of the 9th International Scientific Conference Rural Development 2019, Vytautas Magnus University Agriculture Academy, Akademija, Kaunas district, Lithuania, 26–28 September 2019; Raupelienė, A., Ed.; Vytautas Magnus University Agriculture Academy: Akademija, Kaunas district, Lithuania, 2019; pp. 79–83. [Google Scholar] [CrossRef]
- Combrinck, S.; Regnier, T.; Kamatou, G.P.P. In vitro activity of eighteen essential oils and some major components against common postharvest fungal pathogens of fruit. Ind. Crops Prod. 2011, 33, 344–349. [Google Scholar] [CrossRef]
- Gurjar, M.S.; Ali, S.; Akhtar, M.; Singh, K.S. Efficacy of plant extracts in plant disease management. Agric. Sci. 2012, 3, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Antunes, M.D.C.; Cavaco, A.M. The use of essential oils for postharvest decay control. A review. Flavour Fragr. J. 2010, 25, 351–366. [Google Scholar] [CrossRef]
- Yazdani, F.; Mafi, M.; Farhadi, F.; Tabar-Heidar, K.; Aghapoor, K.; Mohsenzadeh, F.; Darabi, H.R. Supercritical CO2 extraction of essential oil from clove bud: Effect of operation conditions on the selective isolation of eugenol and eugenyl acetate. Z. Naturforsch. B J. Chem. Sci. 2005, 60, 1197–1201. [Google Scholar] [CrossRef]
- Rana, I.S.; Rana, A.S.; Rajak, R.C. Evaluation of antifungal activity in essential oil of the Syzygium aromaticum (L.) by extraction, purification and analysis of its main component eugenol. Braz. J. Microbiol. 2011, 42, 1269–1277. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Costa, W.A.; Pereira, D.S.; Botelho, J.R.S.; Menezes, T.O.A.; Andrade, E.H.A.; Silva, S.H.M.; Filho, A.P.S.S.; Carvalho Junior, R.N. Chemical composition and phytotoxic activity of clove (Syzygium aromaticum) essential oil obtained with supercritical CO2. J. Supercrit. Fluids 2016, 118, 185–193. [Google Scholar] [CrossRef]
- Moghadam, Z.A.; Hosseini, H.; Hadian, Z.; Asgari, B.; Mirmoghtadaie, L.; Mohammadi, A.; Shamloo, E.; Javadi, N.H.S. Evaluation of the antifungal activity of cinnamon, clove, thymes, Zataria multiflora, cumin and caraway essential oils against ochratoxigenic Aspergillus ochraceus. J. Pharm. Res. Int. 2019, 26, 1–16. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, Z.; Cao, D.; Rong, F.; Ding, H.; Zhang, D. Antitermitic and antifungal activities of eugenol and its congeners from the flower buds of Syzygium aromaticum (clove). Ind. Crops Prod. 2015, 77, 780–786. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Chen, H.; Yongjian, Y.; Shi, Z. Antifungal activity of eugenol against Botrytis cinerea. Trop. Plant Pathol. 2010, 35, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Olea, A.F.; Bravo, A.; Martínez, R.; Thomas, M.; Sedan, C.; Espinoza, L.; Zambrano, E.; Carvajal, D.; Silva-Moreno, E.; Carrasco, H. Antifungal activity of eugenol derivatives against Botrytis cinerea. Molecules 2019, 24, 1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melgarejo-Flores, B.G.; Ortega-Ramírez, L.A.; Silva-Espinoza, B.A.; González-Aguilar, G.A.; Miranda, M.R.A.; Ayala-Zavala, J.F. Antifungal protection and antioxidant enhancement of table grapes treated with emulsions, vapors, and coatings of cinnamon leaf oil. Postharvest Biol. Technol. 2013, 86, 321–328. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, S.H.; Kim, N.H.; Lee, S.W.; Jeun, Y.C.; Hong, J.K. Differential inhibitory activities of four plant essential oils on in vitro growth of Fusarium oxysporum f. sp. fragariae causing Fusarium wilt in strawberry plants. Plant Pathol. J. 2017, 33, 582–588. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Q.; Wang, Z.; Cao, H.; Zhang, D. Structure-activity relationships of cinnamaldehyde and eugenol derivatives against plant pathogenic fungi. Ind. Crops Prod. 2017, 97, 388–394. [Google Scholar] [CrossRef]
- Carmello, C.R.; Cardoso, J.C. Effects of plant extracts and sodium hypochlorite on lettuce germination and inhibition of Cercospora longissima in vitro. Sci. Hortic. 2018, 234, 245–249. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, T.; Guo, Z.; Zhang, L.; Mao, L.; Zhang, Y.; Jiang, H. Fumigation and contact activities of 18 plant essential oils on Villosiclava virens, the pathogenic fungus of rice false smut. Sci. Rep. 2019, 9, 7330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Sharma, N.K.; Srivastava, A.; Kataria, A.; Dubey, S.; Sharma, S.; Kundu, B. Clove and lemongrass oil based non-ionic nanoemulsion for suppressing the growth of plant pathogenic Fusarium oxysporum f.sp. lycopersici. Ind. Crops Prod. 2018, 123, 353–362. [Google Scholar] [CrossRef]
- Haddi, K.; Faroni, L.R.A.; Oliveira, E.E. Cinnamon oil. In Green Pesticides Handbook: Essential Oils for Pest Control; Nollet, L.M.L., Rathore, H.S., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 117–150. ISBN 9781498759397. [Google Scholar] [CrossRef]
- Moussa, S.H.; Tayel, A.A.; Alsohim, A.S.; Abdallah, R.R. Botryticidal activity of nanosized silver-chitosan composite and its application for the control of gray mold in strawberry. J. Food Sci. 2013, 78, 1589–1594. [Google Scholar] [CrossRef]
- Pertot, I.; Zasso, R.; Amsalem, L.; Baldessari, M.; Angeli, G.; Elad, Y. Integrating biocontrol agents in strawberry powdery mildew control strategies in high tunnel growing systems. Crop Prot. 2008, 27, 622–631. [Google Scholar] [CrossRef]
- Nikolova, M.; Yordanov, P.; Slavov, S.; Berkov, S. Antifungal activity of some plant extracts against phytopathogenic fungi. J. Biosci. Biotechnol. 2017, 6, 155–161. [Google Scholar]
- Robinson-Boyer, L.; Jeger, M.J.; Xu, X.M.; Jeffries, P. Management of strawberry grey mould using mixtures of biocontrol agents with different mechanisms of action. Biocontrol Sci. Technol. 2009, 19, 1051–1065. [Google Scholar] [CrossRef]
- Xu, X.; Robinson, J.; Jeger, M.; Jeffries, P. Using combinations of biocontrol agents to control Botrytis cinerea on strawberry leaves under fluctuating temperatures. Biocontrol Sci. Technol. 2010, 20, 359–373. [Google Scholar] [CrossRef]
- Bajpai, S.; Shukla, P.S.; Asiedu, S.; Pruski, K.; Prithiviraj, B. A biostimulant preparation of brown seaweed Ascophyllum nodosum suppresses powdery mildew of strawberry. Plant Pathol. J. 2019, 35, 406–416. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Dambrauskienė, E.; Viškelis, P.; Valiuškaitė, A. Efficacy of plant extracts and essential oils for biocontrol of strawberry pathogen Botrytis cinerea. Zemdirb. Agric. 2020, 107, 147–152. [Google Scholar] [CrossRef]
- Rasiukevičiūtė, N.; Rugienius, R.; Šikšnianienė, J.B. Genetic diversity of Botrytis cinerea from strawberry in Lithuania. Zemdirb. Agric. 2018, 105, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Cherkupally, R.; Kota, S.R.; Amballa, H.; Reddy, B.N. In vitro antifungal potential of plant extracts against Fusarium oxysporum, Rhizoctonia solani and Macrophomina phaseolina. Ann. Plant Sci. 2017, 6, 1676–1680. [Google Scholar] [CrossRef] [Green Version]




| Plant Extracts | Cinnamomum cassia | Syzygium aromaticum | ||
|---|---|---|---|---|
| Component | PA 1 (%) | RT 2 | PA (%) | RT |
| α-pinene | 0.47 | 6.694 | 0.26 | 6.695 |
| Camphene | 0.16 | 7.075 | ||
| Benzaldehyde | 0.23 | 7.423 | ||
| β-pinene | 0.16 | 7.803 | ||
| Limonene | 0.15 | 9.217 | ||
| Eucalyptol | 0.73 | 9.279 | 0.36 | 9.285 |
| Linalool | 0.10 | 11.261 | ||
| Borneol | 0.22 | 13.201 | ||
| Terpinen-4-ol | 0.18 | 13.496 | ||
| α-terpineol | 0.28 | 13.894 | ||
| Chavicol | 0.14 | 15.983 | ||
| trans-cinnamaldehyde | 74.67 | 16.561 | ||
| α-cubebene | 0.82 | 18.163 | ||
| Eugenol | 2.55 | 18.478 | 52.88 | 18.787 |
| Cyclosativene | 0.19 | 18.642 | ||
| α-copaene | 3.51 | 18.913 | 0.93 | 18.935 |
| β-elemene | 0.11 | 19.284 | ||
| cis-α-bergamotene | 0.85 | 19.849 | ||
| trans-caryophyllene | 1.13 | 20.033 | 17.80 | 20.168 |
| trans-α-bergamotene | 0.11 | 20.353 | ||
| Coumarin | 6.05 | 20.659 | ||
| α-humulene | 0.20 | 20.887 | 2.00 | 20.922 |
| γ-muurolene | 0.28 | 21.418 | ||
| α-curcumene | 0.10 | 21.512 | ||
| Germacrene D | 0.27 | 21.568 | ||
| β-selinene | 0.11 | 21.697 | ||
| α-muurolene | 0.75 | 21.996 | ||
| β-bisabolene | 0.10 | 22.138 | ||
| 7-epi-α-selinene | 0.13 | 22.456 | ||
| trans-calamenene + eugenyl acetate + cadinene | 1.59 | 22.584 | ||
| Eugenol acetate | 21.95 | 22.822 | ||
| α-calacorene | 0.12 | 23.043 | ||
| Caryophyllene oxide | 0.66 | 24.099 | 0.52 | 24.134 |
| Epicubenol | 0.22 | 25.341 | ||
| T-muurolol | 0.24 | 25.731 | ||
| α-muurolol | 0.11 | 25.828 | ||
| α-cadinol | 0.10 | 26.057 | ||
| Cadalene | 0.11 | 26.516 | ||
| Methyl atrarate | 0.13 | 27.423 | ||
| Hexadecenoic acid | 0.66 | 31.061 | ||
| Squalene | 0.53 | 33.304 | ||
| Other 3 | 1.40 | 1.35 | ||
| Total identified | 98.86% | 99.81% | ||
| Plant Extracts | Radial Colony Growth (mm) at Different Concentrations in μL L−1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 400 | 600 | 800 | 1000 | 1200 | 1400 | 1600 | 1800 | 2000 | 2200 | |
| C. cassia | 34.8 ± 1.8 | 37.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| S. aromaticum | 4.0 ± 4.0 | 43.0 ± 1.5 | 21.8 ± 4.3 | 14.3 ± 2.2 | 15.0 ± 0.5 | 9.8 ± 1.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Control | 39.3 ± 0.7 | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. The Extracts of Cinnamon and Clove as Potential Biofungicides against Strawberry Grey Mould. Plants 2020, 9, 613. https://doi.org/10.3390/plants9050613
Šernaitė L, Rasiukevičiūtė N, Valiuškaitė A. The Extracts of Cinnamon and Clove as Potential Biofungicides against Strawberry Grey Mould. Plants. 2020; 9(5):613. https://doi.org/10.3390/plants9050613
Chicago/Turabian StyleŠernaitė, Lina, Neringa Rasiukevičiūtė, and Alma Valiuškaitė. 2020. "The Extracts of Cinnamon and Clove as Potential Biofungicides against Strawberry Grey Mould" Plants 9, no. 5: 613. https://doi.org/10.3390/plants9050613