Expression of a Hyperthermophilic Cellobiohydrolase in Transgenic Nicotiana tabacum by Protein Storage Vacuole Targeting
Abstract
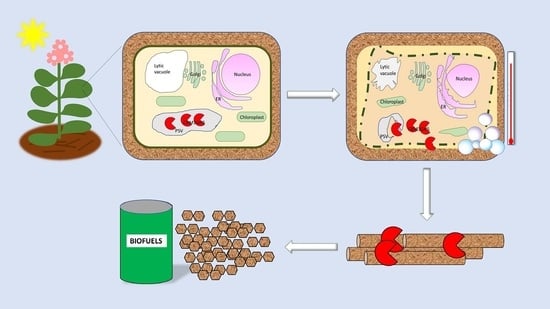
:1. Introduction
2. Results
2.1. Design and Transient Expression of CBM3GH5-HA and CBM3GH5-HA-VAC in N. tabacum
2.2. Stable Expression of CBM3GH5-HA and CBM3GH5-HA-VAC in N. tabacum
2.3. Spatial and Temporal Accumulation Pattern of CBM3GH5-HA-VAC in Transgenic Plants
2.4. Biochemical Characterization of CBM3GH5-HA Purified from the Transgenic Plants
2.5. Leaves and Stems from CBM3GH5-HA-VAC-Expressing Plant Showed an Increased Temperature-Dependent Saccharification Yield
3. Discussion
4. Materials and Methods
4.1. Synthesis In Vitro and Cloning of the Gene Encoding CBM3GH5
4.2. Transient and Stable Expression of CBM3GH5 in N. tabacum by Agrobacterium-Mediated Transformation
4.3. Growth and Selection of Transgenic Tobacco Plants
4.4. Immuno-Decoration Analysis and Activity Assay Using Protein Extract from Transgenic Tobacco Plants
4.5. Gene Expression Analysis in N. tabacum
4.6. Purification of Recombinant Cellobiohydrolase from CBM3GH5-HA-VAC#4-11 Plants
4.7. Saccharification Assay on Plant Materials
4.8. Determination of Total Carbohydrates in the Incubation Medium and in the Leaf Material
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, Q.; Song, J.; Peng, S.; Wang, J.; Qu, G.-Z.; Sederoff, R.R.; Chiang, V.L. Plant biotechnology for lignocellulosic biofuel production. Plant Biotechnol. J. 2014, 12, 1174–1192. [Google Scholar] [CrossRef]
- Benedetti, M.; Locci, F.; Gramegna, G.; Sestili, F.; Savatin, D.-V. Green production and biotechnological applications of cell wall lytic enzymes. Appl. Sci. 2019, 9, 5012. [Google Scholar] [CrossRef] [Green Version]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall–degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef]
- Lagaert, S.; Beliën, T.; Volckaert, G. Plant cell walls: Protecting the barrier from degradation by microbial enzymes. Semin. Cell Dev. Biol. 2009. [Google Scholar] [CrossRef]
- Boudart, G.; Charpentier, M.; Lafitte, C.; Martinez, Y.; Jauneau, A.; Gaulin, E.; Esquerré-Tugayé, M.-T.; Dumas, B. Elicitor activity of a fungal endopolygalacturonase in tobacco requires a functional catalytic site and cell wall localization. Plant Physiol. 2003. [Google Scholar] [CrossRef] [Green Version]
- Benedetti, M.; Pontiggia, D.; Raggi, S.; Cheng, Z.; Scaloni, F.; Ferrari, S.; Ausubel, F.M.; Cervone, F.; De Lorenzo, G. Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns. Proc. Natl. Acad. Sci. USA 2015, 112, 5533–5538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Azevedo Souza, C.; Li, S.; Lin, A.Z.; Boutrot, F.; Grossmann, G.; Zipfel, C.; Somerville, S.C. Cellulose-derived oligomers act as damage-associated molecular patterns and trigger defense-like responses. Plant Physiol. 2017, 173, 2383–2398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Han, C.; Chen, J.; Li, H.; He, K.; Liu, A.; Li, D. Fungal cellulase is an elicitor but its enzymatic activity is not required for its elicitor activity. Mol. Plant Pathol. 2015, 16, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Poinssot, B.; Vandelle, E.; Bentéjac, M.; Adrian, M.; Levis, C.; Brygoo, Y.; Garin, J.; Sicilia, F.; Coutos-Thévenot, P.; Pugin, A. The endopolygalacturonase 1 from Botrytis cinerea activates grapevine defense reactions unrelated to its enzymatic activity. Mol. Plant Microbe Interact. 2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.W.; Klessig, D.F. DAMPs, MAMPs, and NAMPs in plant innate immunity. BMC Plant Biol. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frías, M.; González, M.; González, C.; Brito, N. A 25-residue peptide from Botrytis cinerea xylanase BcXyn11A elicits plant defenses. Front. Plant Sci. 2019, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C. Plant pattern-recognition receptors. Trends Immunol. 2014, 35, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Verrascina, I.; Pontiggia, D.; Locci, F.; Mattei, B.; De Lorenzo, G.; Cervone, F. Four Arabidopsis berberine bridge enzyme-like proteins are specific oxidases that inactivate the elicitor-active oligogalacturonides. Plant J. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capodicasa, C.; Vairo, D.; Zabotina, O.; McCartney, L.; Caprari, C.; Mattei, B.; Manfredini, C.; Aracri, B.; Benen, J.; Knox, J.P.; et al. Targeted modification of homogalacturonan by transgenic expression of a fungal polygalacturonase alters plant growth. Plant Physiol. 2004, 135, 1294–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannoni, M.; Gramegna, G.; Benedetti, M.; Mattei, B. Industrial use of cell wall degrading enzymes: The fine line between production strategy and economic feasibility. Front. Bioeng. Biotechnol. 2020, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.; Bock, R. High-level expression of a suite of thermostable cell wall-degrading enzymes from the chloroplast genome. Plant Mol. Biol. 2011. [Google Scholar] [CrossRef]
- Castiglia, D.; Sannino, L.; Marcolongo, L.; Ionata, E.; Tamburino, R.; De Stradis, A.; Cobucci-Ponzano, B.; Moracci, M.; La Cara, F.; Scotti, N. High-level expression of thermostable cellulolytic enzymes in tobacco transplastomic plants and their use in hydrolysis of an industrially pretreated Arundo donax L. biomass. Biotechnol. Biofuels 2016. [Google Scholar] [CrossRef] [Green Version]
- Harrison, M.D.; Geijskes, J.; Coleman, H.D.; Shand, K.; Kinkema, M.; Palupe, A.; Hassall, R.; Sainz, M.; Lloyd, R.; Miles, S. Accumulation of recombinant cellobiohydrolase and endoglucanase in the leaves of mature transgenic sugar cane. Plant Biotechnol. J. 2011. [Google Scholar] [CrossRef] [Green Version]
- Ziegelhoffer, T.; Will, J.; Austin-Phillips, S. Expression of bacterial cellulase genes in transgenic alfalfa (Medicago sativa L.), potato (Solanum tuberosum L.) and tobacco (Nicotiana tabacum L.). Mol. Breed. 1999, 5, 309–318. [Google Scholar] [CrossRef]
- Dai, Z.; Quesenberry, R.D.; Gao, J.; Hooker, B.S. Expression of Trichoderma reesei exo-cellobiohydrolase I in transgenic tobacco leaves and calli. Appl. Biochem. Biotechnol. 1999, 79, 689–699. [Google Scholar] [CrossRef]
- Jin, S.; Kanagaraj, A.; Verma, D.; Lange, T.; Daniell, H. Release of hormones from conjugates: Chloroplast expression of β-glucosidase results in elevated phytohormone levels associated with significant increase in biomass and protection from aphids or whiteflies conferred by sucrose esters. Plant Physiol. 2011, 155, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Park, J.I.; Kent, M.S.; Datta, S.; Holmes, B.M.; Huang, Z.; Simmons, B.A.; Sale, K.L.; Sapra, R. Enzymatic hydrolysis of cellulose by the cellobiohydrolase domain of CelB from the hyperthermophilic bacterium Caldicellulosiruptor saccharolyticus. Bioresour. Technol. 2011, 102, 5988–5994. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Phillips, T.E.; Hamm, C.A.; Drozdowicz, Y.M.; Rea, P.A.; Maeshima, M.; Rogers, S.W.; Rogers, J.C. The protein storage vacuole: A unique compound organelle. J. Cell Biol. 2001, 155, 991–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, G. Vacuolar storage proteins and the putative vacuolar sorting receptor BP-80 exit the Golgi apparatus of developing pea cotyledons in different transport vesicles. Plant Cell Online 1999. [Google Scholar] [CrossRef] [Green Version]
- Neuhaus, J.M.; Ahl-Goy, P.; Hinz, U.; Flores, S.; Meins, F. High-level expression of a tobacco chitinase gene in Nicotiana sylvestris. Susceptibility of transgenic plants to Cercospora nicotianae infection. Plant Mol. Biol. 1991. [Google Scholar] [CrossRef]
- Neuhaus, J.M.; Sticher, L.; Meins, F.; Boller, T. A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc. Natl. Acad. Sci. USA 1991, 88, 10362–10366. [Google Scholar] [CrossRef] [Green Version]
- Claude, S.J.D.; Marie-Agnès, G.; Catalina, R.; Nadine, P.; Marie-Christine, K.M.; Jean-Marc, N.; Loïc, F.; Véronique, G. Targeting of proConA to the plant vacuole depends on its nine amino-acid C-terminal propeptide. Plant Cell Physiol. 2005. [Google Scholar] [CrossRef] [Green Version]
- Stigliano, E.; Di Sansebastiano, G.-P.; Neuhaus, J.-M. Contribution of chitinase A’s C-terminal vacuolar sorting determinant to the study of soluble protein compartmentation. Int. J. Mol. Sci. 2014, 15, 11030–11039. [Google Scholar] [CrossRef] [Green Version]
- D’Ovidio, R.; Raiola, A.; Capodicasa, C.; Devoto, A.; Pontiggia, D.; Roberti, S.; Galletti, R.; Conti, E.; O’Sullivan, D.; De Lorenzo, G. Characterization of the complex locus of bean encoding polygalacturonase-inhibiting proteins reveals subfunctionalization for defense against fungi and insects. Plant Physiol. 2004. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Kim, S.J.; Vitale, A.; Hwang, I. Identification of the protein storage vacuole and protein targeting to the vacuole in leaf cells of three plant species. Plant Physiol. 2004, 134, 625–639. [Google Scholar] [CrossRef] [Green Version]
- Benedetti, M.; Barera, S.; Longoni, P.; Guardini, Z.; Herrero Garcia, N.; Bolzonella, D.; Lopez-Arredondo, D.; Herrera-Estrella, L.; Goldschmidt-Clermont, M.; Bassi, R. A microalgal-based preparation with synergistic cellulolytic and detoxifying action towards chemical-treated lignocellulose. Plant Biotechnol. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Eamens, A.; Wang, M.-B.; Smith, N.A.; Waterhouse, P.M. RNA silencing in plants: Yesterday, today, and tomorrow. Plant Physiol. 2008, 147, 456–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, A.; Gauthier, A.; Bézier, A.; Poinssot, B.; Joubert, J.-M.; Pugin, A.; Heyraud, A.; Baillieul, F. Elicitor and resistance-inducing activities of beta-1,4 cellodextrins in grapevine, comparison with beta-1,3 glucans and alpha-1,4 oligogalacturonides. J. Exp. Bot. 2007, 58, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Savatin, D.-V.; Sicilia, F.; Gramegna, G.; Cervone, F.; De Lorenzo, G. Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 2013, 4, 49. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; West, C.A. Polygalacturonase from Rhizopus stolonifer, an elicitor of casbene synthetase activity in castor bean (Ricinus communis L.) seedlings. Plant Physiol. 1981, 67, 633–639. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.J. Cellulase elicitor induced accumulation of capsidiol in Capsicum annumm L. suspension cultures. Biotechnol. Lett. 2008, 30, 961–965. [Google Scholar] [CrossRef]
- Hraška, M.; Rakouský, S.; Čurn, V. Tracking of the CaMV-35S promoter performance in GFP transgenic tobacco, with a special emphasis on flowers and reproductive organs, confirmed its predominant activity in vascular tissues. Plant Cell Tissue Organ Cult. 2008. [Google Scholar] [CrossRef]
- Harrison, M.D.; Geijskes, R.J.; Lloyd, R.; Miles, S.; Palupe, A.; Sainz, M.B.; Dale, J.L. Recombinant cellulase accumulation in the leaves of mature, vegetatively propagated transgenic sugarcane. Mol. Biotechnol. 2014, 56, 795–802. [Google Scholar] [CrossRef]
- Herman, E.; Larkins, B. Protein storage bodies and vacuoles. Plant Cell 1999, 11, 601–614. [Google Scholar] [CrossRef] [Green Version]
- Patchett, M.L.; Neal, T.L.; Schofield, L.R.; Strange, R.C.; Daniel, R.M.; Morgan, H.W. Heat treatment purification of thermostable cellulase and hemicellulase enzymes expressed in E. coli. Enzym. Microb. Technol. 1989, 11, 113–115. [Google Scholar] [CrossRef]
- Lionetti, V.; Francocci, F.; Ferrari, S.; Volpi, C.; Bellincampi, D.; Galletti, R.; D’Ovidio, R.; De Lorenzo, G.; Cervone, F. Engineering the cell wall by reducing de-methyl-esterified homogalacturonan improves saccharification of plant tissues for bioconversion. Proc. Natl. Acad. Sci. USA 2010, 107, 616–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gama, R.; Van Dyk, J.S.; Pletschke, B.I. Optimisation of enzymatic hydrolysis of apple pomace for production of biofuel and biorefinery chemicals using commercial enzymes. 3 Biotech 2015, 5, 1075–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, J.K.; Li, X.; Bonawitz, N.D.; Chapple, C. Emerging strategies of lignin engineering and degradation for cellulosic biofuel production. Curr. Opin. Biotechnol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Bindschedler, L.V.; Tuerck, J.; Maunders, M.; Ruel, K.; Petit-Conil, M.; Danoun, S.; Boudet, A.M.; Joseleau, J.P.; Bolwell, G.P. Modification of hemicellulose content by antisense down-regulation of UDP-glucuronate decarboxylase in tobacco and its consequences for cellulose extractability. Phytochemistry 2007, 68, 2635–2648. [Google Scholar] [CrossRef] [PubMed]
- Biswal, A.K.; Atmodjo, M.A.; Li, M.; Baxter, H.L.; Yoo, C.G.; Pu, Y.; Lee, Y.; Mazarei, M.; Black, I.M.; Zhang, J.; et al. Sugar release and growth of biofuel crops are improved by downregulation of pectin biosynthesis. Nat. Biotechnol. 2018, 36, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Greene, E.R.; Himmel, M.E.; Beckham, G.T.; Tan, Z. Glycosylation of cellulases. Adv. Carbohydr. Chem. Biochem. 2015, 72, 63–112. [Google Scholar]
- Marin Viegas, V.S.; Ocampo, C.G.; Petruccelli, S. Vacuolar deposition of recombinant proteins in plant vegetative organs as a strategy to increase yields. Bioengineered 2017. [Google Scholar] [CrossRef] [Green Version]
- Klose, H.; Günl, M.; Usadel, B.; Fischer, R.; Commandeur, U. Ethanol inducible expression of a mesophilic cellulase avoids adverse effects on plant development. Biotechnol. Biofuels 2013, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Zuo, J.; Niu, Q.-W.; Chua, N.-H. An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 2000, 24, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Tomassetti, S.; Pontiggia, D.; Verrascina, I.; Reca, I.B.; Francocci, F.; Salvi, G.; Cervone, F.; Ferrari, S. Controlled expression of pectic enzymes in Arabidopsis thaliana enhances biomass conversion without adverse effects on growth. Phytochemistry 2015, 112, 221–230. [Google Scholar] [CrossRef]
- Mir, B.A.; Mewalal, R.; Mizrachi, E.; Myburg, A.A.; Cowan, D.A. Recombinant hyperthermophilic enzyme expression in plants: A novel approach for lignocellulose digestion. Trends Biotechnol. 2014, 32, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, B.A.; Myburg, A.A.; Mizrachi, E.; Cowan, D.A. In planta expression of hyperthermophilic enzymes as a strategy for accelerated lignocellulosic digestion. Sci. Rep. 2017, 7, 11462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarmiento, F.; Peralta, R.; Blamey, J.M. Cold and hot extremozymes: Industrial relevance and current trends. Front. Bioeng. Biotechnol. 2015, 3, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, D.M.; Morag, E.; Lamed, R.; Bayer, E.A.; Hazlewood, G.P.; Gilbert, H.J. Identification of the cellulose-binding domain of the cellulosome subunit S1 from Clostridium thermocellum YS. FEMS Microbiol. Lett. 1992. [Google Scholar] [CrossRef]
- Carrard, G.; Koivula, A.; Soderlund, H.; Beguin, P. Cellulose-binding domains promote hydrolysis of different sites on crystalline cellulose. Proc. Natl. Acad. Sci. USA 2000. [Google Scholar] [CrossRef] [Green Version]
- Faè, M.; Accossato, S.; Cella, R.; Fontana, F.; Goldschmidt-Clermont, M.; Leelavathi, S.; Reddy, V.S.; Longoni, P. Comparison of transplastomic Chlamydomonas reinhardtii and Nicotiana tabacum expression system for the production of a bacterial endoglucanase. Appl. Microbiol. Biotechnol. 2017, 101, 4085–4092. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Betterle, N.; Natali, A.; Bassi, R.; Dall’Osto, L. Design of a highly thermostable hemicellulose-degrading blend from Thermotoga neapolitana for the treatment of lignocellulosic biomass. J. Biotechnol. 2019, 296, 42–52. [Google Scholar] [CrossRef]
- Puigbò, P.; Guzmán, E.; Romeu, A.; Garcia-Vallvé, S. OPTIMIZER: A web server for optimizing the codon usage of DNA sequences. Nucleic Acids Res. 2007. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, R.; Qi, M. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J. 2000, 22, 543–551. [Google Scholar] [CrossRef]
- Horsch, R.B.; Fry, J.E.; Hoffmann, N.L.; Eichholtz, D.; Rogers, S.G.; Fraley, R.T. A simple and general method for transferring genes into plants. Science 1985, 217, 1229–1231. [Google Scholar]
- Rogers, S.G.; Horsch, R.B.; Fraley, R.T. Gene transfer in plants: Production of transformed plants using Ti plasmid vectors. Methods Enzymol. 1986, 118, 627–640. [Google Scholar]
- Lever, M. A new reaction for colorimetric determination of carbohydrates. Anal. Biochem. 1972, 47, 273–279. [Google Scholar] [CrossRef]
- Schmidt, G.W.; Delaney, S.K. Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol. Genet. Genomics 2010. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetti, M.; Vecchi, V.; Guardini, Z.; Dall’Osto, L.; Bassi, R. Expression of a Hyperthermophilic Cellobiohydrolase in Transgenic Nicotiana tabacum by Protein Storage Vacuole Targeting. Plants 2020, 9, 1799. https://doi.org/10.3390/plants9121799
Benedetti M, Vecchi V, Guardini Z, Dall’Osto L, Bassi R. Expression of a Hyperthermophilic Cellobiohydrolase in Transgenic Nicotiana tabacum by Protein Storage Vacuole Targeting. Plants. 2020; 9(12):1799. https://doi.org/10.3390/plants9121799
Chicago/Turabian StyleBenedetti, Manuel, Valeria Vecchi, Zeno Guardini, Luca Dall’Osto, and Roberto Bassi. 2020. "Expression of a Hyperthermophilic Cellobiohydrolase in Transgenic Nicotiana tabacum by Protein Storage Vacuole Targeting" Plants 9, no. 12: 1799. https://doi.org/10.3390/plants9121799