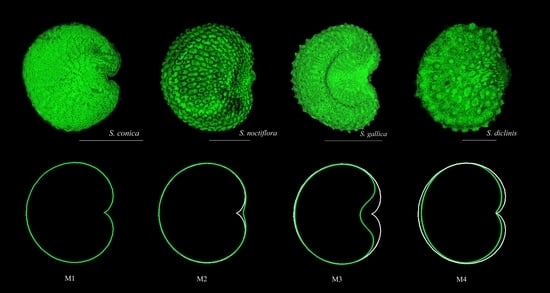
Seed Morphology in Silene Based on Geometric Models
Abstract
:1. Introduction
2. Results
2.1. General Morphological Description: Size and Shape in the Seeds of Silene Species
2.2. Structural Aspects
2.2.1. Seed Surface
2.2.2. Other Properties of Seeds
2.3. Morphological Description of Silene Species by Similarity with Geometric Models
2.3.1. Similarity with the Cardioid. J Index Values
2.3.2. Comparison between Subgenera
2.3.3. Similarity with Other Figures Related the Cardioid
2.4. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Species of Silene
4.2. Seed Images
4.3. Surface Characteristics and other Structural Properties of Seeds
4.4. General Morphological Description by Image Analysis
4.5. Comparison with Geometric Models: Calculation of the J Index
4.6. Statistical Analysis
Multivariate Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A






| MANOVA (Multi Analysis of Variance) | ||||||
|---|---|---|---|---|---|---|
| Df | Pillai | Approx. F num. | Df | Den DF | Pr (>F) | |
| (Intercept) | 1 | 1 | 1,440,064 | 3 | 21 | <2.2 × 10−16 *** |
| Subgenus | 1 | 0.42024 | 5 | 3 | 21 | 0.008473 ** |
| Residuals | 23 | |||||
References
- Perveen, A.; Qaiser, M. Pollen flora of Pakistan-LI- Caryophyllaceae. Pak. J. Bot. 2006, 38, 901–915. [Google Scholar]
- Hernández-Ledesma, P.; Berendsohn, W.G.; Borsch, T.; Von Mering, S.; Akhani, H.; Arias, S.; Castañeda-Noa, I.; Eggli, U.; Eriksson, R.; Flores-Olvera, H.; et al. A taxonomic backbone for the global synthesis of species diversity in the angiosperm order Caryophyllales. Willdenowia 2015, 45, 281. [Google Scholar] [CrossRef] [Green Version]
- Frajman, B.; Schönswetter, P.; Weiss-Schneeweiss, H.; Oxelman, B. Origin and Diversification of South American Polyploid Silene Sect. Physolychnis (Caryophyllaceae) in the Andes and Patagonia. Front. Genet. 2018, 9, 639. [Google Scholar] [CrossRef]
- Christenhusz, M.J.; Fay, M.F.; Chase, M.W. Plants of the World: An Illustrated Encyclopedia of Vascular Plants; University of Chicago Press: Chicago, IL, USA, 2017. [Google Scholar]
- Dadandi, M.Y.; Yildiz, K. Seed morphology of some Silene L. (Caryophyllaceae) species collected from Turkey. Turk. J. Bot. 2015, 39, 280–297. [Google Scholar] [CrossRef]
- Fawzi, N.M.; Fawzy, A.M.; Mohamed, A.A.-H.A. Seed Morphological Studies on Some Species of Silene L. (Caryophyllaceae). Int. J. Bot. 2010, 6, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Greuter, W. Silene (Caryophyllaceae) in Greece: A subgeneric and sectional classification. TAXON 1995, 44, 543–581. [Google Scholar] [CrossRef]
- Oxelman, B.; Rautenberg, A.; Thollesson, M.; Larsson, A.; Frajman, B.; Eggens, F. Sileneae Taxonomy and Systematics. 2013. Available online: http://www.sileneae.info (accessed on 27 May 2020).
- Ullah, F.; Ayaz, A.; Saqib, S.; Zaman, W.; Butt, M.A.; Ullah, A. Silene conoidea L.: A Review on its Systematic, Ethnobotany and Phytochemical profile. Plant Sci. Today 2019, 6, 373–382. [Google Scholar] [CrossRef]
- Mira, S.; González-Benito, M.E.; Ibars, A.M.; Estrelles, E. Dormancy release and seed ageing in the endangered species Silene diclinis. Biodivers. Conserv. 2010, 20, 345–358. [Google Scholar] [CrossRef]
- Alonso-Vargas, M.Á.; Guilló, A.; Pérez-Botella, J.; Crespo, M.B.; Juan, A. Genetic assessment of population restorations of the critically endangered Silene hifacensis in the Iberian Peninsula. J. Nat. Conserv. 2014, 22, 532–538. [Google Scholar] [CrossRef] [Green Version]
- Ferrer-Gallego, P.P.; Ferrando-Pardo, I.; Laguna, E.; Pérez-Rovira, P.; Jiménez, J. Recovery plans of threatened flora of the Valencian Community. Results after three years since the approval of the legal normative. Flora Montiberica 2019, 73, 48–61. [Google Scholar]
- López-Pujol, J.; Font, J.; Simon, J.; Blanché, C. Can the preservation of historical relicts permit the conservation of endangered plant species? The case of Silene sennenii (Caryophyllaceae). Conserv. Genet. 2006, 8, 903–912. [Google Scholar] [CrossRef]
- Jafari, F.; Zarre, S.; Gholipour, A.; Eggens, F.; Rabeler, R.K.; Oxelman, B. A new taxonomic backbone for the infrageneric classification of the species-rich genus Silene (Caryophyllaceae). TAXON 2020, 69, 337–368. [Google Scholar] [CrossRef]
- Wofford, B.E. External Seed Morphology of Arenaria (Caryophyllaceae) of the Southeastern United States. Syst. Bot. 1981, 6, 126. [Google Scholar] [CrossRef]
- Wyatt, R. Intraspecific Variation in Seed Morphology of Arenaria uniflora (Caryophyllaceae). Syst. Bot. 1984, 9, 423. [Google Scholar] [CrossRef]
- Amini, E.; Zarre, S.; Assadi, M. Seed micro-morphology and its systematic significance in Gypsophila (Caryophyllaceae) and allied genera. Nord. J. Bot. 2011, 29, 660–669. [Google Scholar] [CrossRef]
- Kovtonyuk, N.K. The structure of seed Surface and the systematics of the Siberian Gypsophila species (Caryophyllaceae). Bot. Z. 1994, 79, 48–51. [Google Scholar]
- Minuto, L.; Fior, S.; Roccotiello, E.; Casazza, G. Seed morphology in Moehringia L. and its taxonomic significance in comparative studies within the Caryophyllaceae. Plant Syst. Evol. 2006, 262, 189–208. [Google Scholar] [CrossRef]
- Kaplan, A.; Çölgeçen, H.; Büyükkartal, H.N. Seed morphology and histology of some Paronychia taxa (Caryophyllaceae) from Turkey. Bangladesh J. Bot. 2009, 38, 171–176. [Google Scholar] [CrossRef]
- Crow, G.E. The Systematic Significance of Seed Morphology in Sagina (Caryophyllaceae) Under Scanning Electron Microscopy. Brittonia 1979, 31, 52–63. [Google Scholar] [CrossRef]
- Volponi, C.R. Stellaria cuspidata (Caryophyllaceae) and some related species in the Andes. Willdenowia 1993, 23, 193–209. [Google Scholar]
- Poyraz, I.E.; Ataşlar, E. Pollen and seed morphology of Velezia L. (Caryophyllaceae) genus in Turkey. Turk. J. Bot. 2010, 34, 179–190. [Google Scholar]
- Hong, S.-P.; Han, M.-J.; Kim, K.-J. Systematic significance of seed coat morphology in Silene L. s. str. (Sileneae-Caryophyllaceae) from Korea. J. Plant Biol. 1999, 42, 146–150. [Google Scholar] [CrossRef]
- Rohrbach, P. Monographic der Gattung Silene; Verlag von Engelmann: Leipzig, Germany, 1869; pp. 1–249. [Google Scholar]
- Chowdhuri, P.K. Studies in the genus Silene. Notes. R. Bot. Gard. 1957, 22, 221–287. [Google Scholar]
- Ghazanfar, S.A. Seed characters as diagnostic in the perennial sections of the genus Silene (Family Caryophyllaceae). Pak. J. Bot. 1983, 15, 7–12. [Google Scholar]
- Melzheimer, V. On the taxonomic position of Silene thebana (Caryophyllaceae). Plant Syst. Evol. 1987, 155, 251–256. [Google Scholar] [CrossRef]
- Bojňanský, V.; Fargašová, A. Atlas of Seeds and Fruits of Central and East-European Flora: The Carpathian Mountains Region; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–954. [Google Scholar]
- Yildiz, K.; Cirpici, A. Seed morphological studies of Silene L. from Turkey. J. Bot. 1998, 30, 173–188. [Google Scholar]
- Hoseini, E.; Ghahremaninejad, F.; Assadi, M.; Edalatiyan, M.N. Seed micromorphology and its implication in subgeneric classification of Silene (Caryophyllaceae, Sileneae). Flora Morphol. Distrib. Funct. Ecol. Plants 2017, 228, 31–38. [Google Scholar] [CrossRef]
- Cervantes, E.; Gómez, J.J.M. Seed Shape Description and Quantification by Comparison with Geometric Models. Horticulturae 2019, 5, 60. [Google Scholar] [CrossRef] [Green Version]
- Cervantes, E.; Martín, J.J.; Saadaoui, E. Updated Methods for Seed Shape Analysis. Science 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cervantes, E.; Martín, J.J.; Ardanuy, R.; De Diego, J.G.; Tocino, Á. Modeling the Arabidopsis seed shape by a cardioid: Efficacy of the adjustment with a scale change with factor equal to the Golden Ratio and analysis of seed shape in ethylene mutants. J. Plant Physiol. 2010, 167, 408–410. [Google Scholar] [CrossRef]
- Cervantes, E.; Martín, J.J.; Chan, P.K.; Gresshoff, P.M.; Tocino, Á. Seed shape in model legumes: Approximation by a cardioid reveals differences in ethylene insensitive mutants of Lotus japonicus and Medicago truncatula. J. Plant Physiol. 2012, 169, 1359–1365. [Google Scholar] [CrossRef] [Green Version]
- Cervantes, E.; Saadaoui, E.; Tocino, Á.; Martín-Gómez, J.J. Seed Shape Quantification in the Model Legumes: Methods and Applications. In The Model Legume Medicago truncatula; Wiley: Hoboken, NJ, USA, 2020; pp. 92–98. [Google Scholar]
- Saadaoui, E.; Martín, J.J.; Cervantes, E. Seed morphology in Tunisian wild populations of Capparis spinosa L. Acta Biol. Cracov Ser. Bot. 2013, 55, 99–106. [Google Scholar] [CrossRef]
- Martín-Gómez, J.J.; Rewicz, A.; Cervantes, E. Seed Shape Diversity in families of the Order Ranunculales. Phytotaxa 2019, 425, 193–207. [Google Scholar] [CrossRef]
- Gómez, J.J.M.; Del Pozo, D.G.; Cervantes, E. Seed Shape Quantification in the Malvaceae Reveals Cardioid-Shaped Seeds Predominantly in Herbs. Botanica 2019, 25, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Cervantes, E.; Martín-Gómez, J.J. Seed shape quantification in the order Cucurbitales. Mod. Phytomorphol. 2018, 12, 1–13. [Google Scholar] [CrossRef]
- Saadaoui, E.; Martín, J.J.; Bouazizi, R.; Ben Romdhane, C.; Grira, M.; Abdelkabir, S.; Khouja, M.L.; Cervantes, E. Phenotypic variability and seed yield of Jatropha curcas L. introduced to Tunisia. Acta Bot. Mex. 2015, 1, 119. [Google Scholar] [CrossRef] [Green Version]
- Gómez, J.J.M.; Saadaoui, E.; Cervantes, E. Seed Shape of Castor Bean (Ricinus communis L.) Grown in Different Regions of Tunisia. J. Agric. Ecol. Res. Int. 2016, 8, 1–11. [Google Scholar] [CrossRef]
- Gómez, J.J.M.; Rewicz, A.; Goriewa-Duba, K.; Wiwart, M.; Tocino, A.; Cervantes, E.; Gómez, M.; Duba, G. Morphological Description and Classification of Wheat Kernels Based on Geometric Models. Agronomy 2019, 9, 399. [Google Scholar] [CrossRef] [Green Version]
- Gómez, J.J.M.; Del Pozo, D.G.; Ucchesu, M.; Bacchetta, G.; De Santamaría, F.C.S.; Tocino, A.; Cervantes, E. Seed Morphology in the Vitaceae Based on Geometric Models. Agronomy 2020, 10, 739. [Google Scholar] [CrossRef]
- Del Pozo, D.G.; Gómez, J.J.M.; Tocino, A.; Cervantes, E. Seed Geometry in the Arecaceae. Horticulturae 2020, 6, 64. [Google Scholar] [CrossRef]
- Brazeau, M.D.; Guillerme, T.; Smith, M.R. An algorithm for Morphological Phylogenetic Analysis with Inapplicable Data. Syst. Biol. 2019, 68, 619–631. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.Y.; Palci, A. Morphological Phylogenetics in the Genomic Age. Curr. Biol. 2015, 25, R922–R929. [Google Scholar] [CrossRef] [Green Version]
- Sukhorukov, A.P.; Nilova, M.V.; Krinitsina, A.A.; Zaika, M.A.; Erst, A.S.; Shepherd, K.A. Molecular phylogenetic data and seed coat anatomy resolve the generic position of some critical Chenopodioideae (Chenopodiaceae—Amaranthaceae) with reduced perianth segments. PhytoKeys 2018, 109, 103–128. [Google Scholar] [CrossRef] [Green Version]
- Slancarova, V.; Zdanska, J.; Janousek, B.; Talianova, M.; Zschach, C.; Zluvova, J.; Široký, J.; Kovacova, V.; Blavet, H.; Danihelka, J.; et al. Evolution of Sex Determination Systems with Heterogametic Males and Females In Silene. Evolution 2013, 67, 3669–3677. [Google Scholar] [CrossRef]
- Bernasconi, G.; Antonovics, J.; Biere, A.L.; Charlesworth, D.; Delph, L.F.; Filatov, D.; Giraud, T.; Hood, M.; Marais, G.A.B.; McCauley, D.W.; et al. Silene as a model system in ecology and evolution. Heredity 2009, 103, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.R. Conservation Assessment for Ovate Catchfly (Silene ovata) Pursh. USDA Forest Service, Eastern Region. Available online: https://core.ac.uk/reader/4819420 (accessed on 12 September 2020).
- Zumajo-Cardona, C.; Ambrose, B.A. Phylogenetic analyses of key developmental genes provide insight into the complex evolution of seeds. Mol. Phylogenetics Evol. 2020, 147, 106778. [Google Scholar] [CrossRef]
- Ghimire, B.; Suh, Y.; Soltis, U.E.; Heo, K. Phylogenetic relationships of Coreanomecon (Papaveraceae: Chelidonioideae) inferred from seed morphology and nrITS sequence data. Nord. J. Bot. 2019, 37, 211–225. [Google Scholar] [CrossRef]
- Stull, G.W.; Johnson, D.M.; Murray, N.A.; Couvreur, T.L.P.; Reeger, J.E.; Roy, C.M. Plastid and Seed Morphology Data Support a Revised Infrageneric Classification and an African Origin of the Pantropical Genus Xylopia (Annonaceae). Syst. Bot. 2017, 42, 211–225. [Google Scholar] [CrossRef]
- Bocquet, G. Revisio Physolychnidum (Silene sect. Physolychnis). Phanerogamarum Monographiae 1; Lehre J. Cramer: Berlin, Germany, 1969. [Google Scholar]
- eFloras. 2008. Available online: http://www.efloras.org (accessed on 24 November 2020).
- Buide, M.L. Pollination Ecology of Silene acutifolia (Caryophyllaceae): Floral Traits Variation and Pollinator Attraction. Ann. Bot. 2005, 97, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Eflorasys. Available online: http://eflorasys.univ-lorraine.fr/index.php/en/species/view/123446 (accessed on 7 September 2020).
- Silene conica. Available online: https://en.wikipedia.org/wiki/Silene_conica (accessed on 15 September 2020).
- Montesinos, D.; Güemes, J. Silenediclinis. The IUCN Red List of Threatened Species 2006. Available online: http://dx.doi.org/10.2305/IUCN.UK.2006.RLTS.T61640A12531397.en (accessed on 7 September 2020).
- Datenbank Biologisch-Ökologischer Merkmale der Flora von Deutschland. Available online: https://www.ufz.de/biolflor/ (accessed on 7 September 2020).
- Anthos: Sistema de Información Sobre las Plantas de España. Available online: http://www.anthos.es/ (accessed on 7 September 2020).
- Stroh, P.A. Silene Otites (L.) Wibel. Spanish Catchfly. Species Account; Botanical Society of Britain and Ireland: Durham, UK, 2015; Available online: https://bsbi.org/wp-content/uploads/dlm_uploads/Silene_otites_species_account.pdf (accessed on 7 September 2020).
- Silene uniflora. Available online: https://en.wikipedia.org/wiki/Silene_uniflora (accessed on 15 September 2020).
- Floraweb. The Federal Agency for Nature Conservation. Das Bundesamt für Naturschutz. 2020. Available online: http://www.floraweb.de/ (accessed on 7 September 2020).
- Plants of the World Online. Available online: http://www.plantsoftheworldonline.org/ (accessed on 7 September 2020).
- Sileneae Classification. Available online: http://www.sileneae.info/classification.php (accessed on 26 September 2020).
- Ferreira, T.; Rasband, W. ImageJ User Guide-IJ1.46r. 2012. Available online: http://imagej.nih.gov/ij/docs/guide (accessed on 7 September 2020).
- Zdilla, M.J.; Hatfield, S.A.; McLean, K.A.; Cyrus, L.M.; Laslo, J.M.; Lambert, H.W. Circularity, Solidity, Axes of a Best Fit Ellipse, Aspect Ratio, and Roundness of the Foramen Ovale. J. Craniofac. Surg. 2016, 27, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Weisstein, E.W. Cardioid. From MathWorld—A Wolfram Web Resource. Available online: https://mathworld.wolfram.com/Cardioid.html (accessed on 7 September 2020).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B Stat. Methodol. 1964, 26, 211–243. [Google Scholar] [CrossRef]













| Species | n | A | P | L | W | AR | C | R |
|---|---|---|---|---|---|---|---|---|
| S. acutifolia | 40 | 1.18 de | 4.55 de | 1.35 efg | 1.15 d | 1.18 cde | 0.72 de | 0.85 bcd |
| (0.14) | (0.32) | (0.08) | (0.07) | (0.06) | (0.03) | (0.04) | ||
| S. conica | 40 | 0.52 i | 2.80 i | 0.89 j | 0.75 g | 1.18 cde | 0.83 a | 0.85 bcd |
| (0.03) | (0.08) | (0.03) | (0.02) | (0.04) | (0.02) | (0.03) | ||
| S. diclinis | 40 | 1.73 b | 5.28 b | 1.65 b | 1.36 b | 1.21 bcd | 0.78 bc | 0.83 cde |
| (0.14) | (0.24) | (0.07) | (0.06) | (0.05) | (0.03) | (0.04) | ||
| S. dioica Chk | 40 | 1.30 c | 5.30 c | 1.42 cd | 1.21 c | 1.16 e | 0.59 f | 0.86 b |
| (0.17) | (0.59) | (0.10) | (0.09) | (0.04) | (0.08) | (0.03) | ||
| S. dioica Pol | 40 | 0.94 h | 4.07 h | 1.20 i | 1.02 f | 1.18 de | 0.71 de | 0.85 bc |
| (0.12) | (0.37) | (0.08) | (0.06) | (0.05) | (0.05) | (0.03) | ||
| S. latifolia Chk | 40 | 1.15 gh | 4.52 gh | 1.33 hi | 1.12 f | 1.18 cde | 0.71 ab | 0.85 bcd |
| (0.14) | (0.43) | (0.09) | (0.07) | (0.05) | (0.06) | (0.03) | ||
| S. latifolia Pol 1 | 40 | 1.07 fg | 4.29 fg | 1.29 gh | 1.06 ef | 1.21 abc | 0.74 d | 0.82 def |
| (0.16) | (0.42) | (0.09) | (0.09) | (0.03) | (0.04) | (0.02) | ||
| S. latifolia Pol 2 | 40 | 1.26 cd | 4.67 cd | 1.41 cde | 1.14 d | 1.22 ab | 0.73 d | 0.82 ef |
| (0.19) | (0.33) | (0.10) | (0.08) | (0.03) | (0.05) | (0.02) | ||
| S. latifolia Pol 3 | 40 | 1.02 ef | 3.97 ef | 1.23 fg | 1.04 d | 1.18 de | 0.80 de | 0.85 bc |
| (0.18) | (0.31) | (0.11) | (0.09) | (0.03) | (0.04) | (0.03) | ||
| S. noctiflora | 40 | 1.28 cd | 4.55 cd | 1.36 def | 1.21 c | 1.12 f | 0.78 c | 0.90 a |
| (0.11) | (0.21) | (0.06) | (0.05) | (0.04) | (0.02) | (0.03) | ||
| S. pendula | 40 | 1.14 ef | 4.48 ef | 1.34 efg | 1.11 de | 1.20 bcde | 0.71 de | 0.84 bcde |
| (0.10) | (0.23) | (0.07) | (0.07) | (0.08) | (0.03) | (0.05) | ||
| S. uniflora | 40 | 1.31 c | 4.51 c | 1.43 c | 1.16 cd | 1.25 a | 0.80 ab | 0.80 f |
| (0.13) | (0.23) | (0.09) | (0.06) | (0.07) | (0.01) | (0.04) | ||
| S. viscosa | 40 | 0.51 i | 3.19 i | 0.89 j | 0.75 g | 1.17 de | 0.63 f | 0.85 bc |
| (0.05) | (0.18) | (0.05) | (0.04) | (0.04) | (0.04) | (0.03) | ||
| S. vulgaris | 40 | 1.20 cde | 4.67 cde | 1.37 cdef | 1.14 d | 1.20 bcd | 0.69 e | 0.83 cde |
| (0.15) | (0.36) | (0.09) | (0.08) | (0.06) | (0.04) | (0.04) | ||
| S. zawadzkii | 40 | 2.02 a | 5.74 a | 1.79 a | 1.46 a | 1.23 ab | 0.77 c | 0.81 ef |
| (0.24) | (0.36) | (0.10) | (0.08) | (0.06) | (0.02) | (0.04) |
| Species | n | A | P | L | W | AR | C | R |
|---|---|---|---|---|---|---|---|---|
| S. colpophylla | 40 | 0.68 c | 3.44 de | 1.08 c | 0.82 d | 1.35 a | 0.72 e | 0.74 c |
| (0.07) | (0.18) | (0.05) | (0.06) | (0.08) | (0.02) | (0.04) | ||
| S. gallica | 40 | 0.53 d | 2.97 e | 0.93 e | 0.74 f | 1.26 b | 0.76 cd | 0.79 c |
| (0.05) | (0.17) | (0.05) | (0.04) | (0.05) | (0.03) | (0.03) | ||
| S. italica | 40 | 0.65 c | 3.21 e | 1.01 d | 0.83 d | 1.21 cd | 0.79 a | 0.83 ab |
| (0.07) | (0.16) | (0.05) | (0.05) | (0.07) | (0.01) | (0.05) | ||
| S. mellifera | 40 | 0.99 a | 3.95 c | 1.25 ab | 1.01 b | 1.25 bc | 0.79 ab | 0.80 bc |
| (0.21) | (0.40) | (0.13) | (0.12) | (0.07) | (0.03) | (0.04) | ||
| S. nutans Chk | 40 | 1.06 a | 4.25 b | 1.3 a | 1.06 a | 1.19 cd | 0.73 d | 0.81 ab |
| (0.12) | (0.24) | (0.05) | (0.08) | (0.06) | (0.04) | (0.05) | ||
| S. nutans Pol | 40 | 1.07 a | 4.22 b | 1.29 a | 1.07 a | 1.21 cd | 0.75 d | 0.83 ab |
| (0.13) | (0.23) | (0.07) | (0.07) | (0.05) | (0.03) | (0.04) | ||
| S. otites | 40 | 0.41 d | 2.60 f | 0.81 e | 0.66 f | 1.24 bcd | 0.76 cd | 0.81 abc |
| (0.06) | (0.20) | (0.07) | (0.05) | (0.07) | (0.02) | (0.05) | ||
| S. saxifraga | 40 | 0.78 b | 3.52 d | 1.11 c | 0.92 c | 1.20 d | 0.79 a | 0.84 a |
| (0.11) | (0.27) | (0.08) | (0.07) | (0.07) | (0.02) | (0.04) | ||
| S. schafta | 40 | 0.82 b | 4.79 a | 1.22 b | 0.93 c | 1.34 a | 0.45 h | 0.75 c |
| (0.11) | (0.59) | (0.09) | (0.07) | (0.06) | (0.06) | (0.03) | ||
| S. tatarica | 40 | 0.54 d | 2.96 e | 0.93 e | 0.76 ef | 1.25 bc | 0.77 bc | 0.80 bc |
| (0.05) | (0.14) | (0.04) | (0.04) | (0.06) | (0.02) | (0.04) | ||
| S. wolgensis | 40 | 0.57 d | 3.21 e | 0.97 de | 0.80 de | 1.23 bcd | 0.70 f | 0.82 abc |
| (0.05) | (0.18) | (0.05) | (0.04) | (0.07) | (0.03) | (0.05) |
| Subg. | Species | Symmetry | Dorsal Face | Ridge | Hilium | ||
|---|---|---|---|---|---|---|---|
| Plane | Canaliculate | Almost None | Almost All | ||||
| Behenantha | S. acutifolia Link ex Rohrb. | - | + | - | - | + | - |
| S. conica L. | + | - | + | - | + | - | |
| S. diclinis (Lag.) M.Laínz | + | - | - | - | - | - | |
| S. dioica (L.) Clairv. | + | - | - | - | - | + | |
| S. latifolia Poir. | + | - | - | - | - | - | |
| S. noctiflora L. | + | - | - | - | - | + | |
| S. pendula L. | + | + | - | - | - | - | |
| S. uniflora Roth | + | + | - | -* | - | - | |
| S. viscosa (L.) Pers. | + | - | - | - | - | - | |
| S. vulgaris (Moench) Garcke | + | + | - | - | - | + | |
| S. zawadzkii Herbich | - | + | - | -* | - | + | |
| Silene | S. colpophylla Wrigley | - | + | + | - | + | - |
| S. gallica L. | + | - | + | + | + | - | |
| S. italica (L.) Pers. | + | + | + | - | + | - | |
| S. mellifera Boiss. and Reut. | - | + | - | - | + | - | |
| S. nutans L. | + | + | - | -* | - | - | |
| S. otites (L.) Wibel | - | + | + | - | - | - | |
| S. saxifraga L. | - | + | + | - | - | - | |
| S. schafta S.G.Gmel. | - | + | - | - | + | - | |
| S. tatarica (L.) Pers. | - | + | + | - | + | - | |
| S. wolgensis (Hornem.) Otth | - | + | + | - | + | - | |
| Species | n | J Index (Cardioid) | Min | Max | Standard Dev. |
|---|---|---|---|---|---|
| S. acutifolia | 40 | 91.2 bcdef | 86 | 94.1 | 1.84 |
| S. conica | 40 | 92.1 bcd | 89 | 94.6 | 1.28 |
| S. diclinis | 40 | 90.3 ef | 83 | 93.6 | 2.63 |
| S. dioica Chk | 40 | 91.5 bcde | 88 | 94.1 | 1.18 |
| S. dioica Pol | 40 | 91.7 bcde | 85 | 95.8 | 1.34 |
| S. latifolia Chk | 40 | 92.5 a | 83 | 93.5 | 1.97 |
| S. latifolia Pol 1 | 40 | 92.2 bcd | 89 | 94 | 0.88 |
| S. latifolia Pol 2 | 40 | 92.4 abc | 91 | 95.2 | 1.18 |
| S. latifolia Pol 2 | 40 | 93.5 abc | 87 | 95.8 | 1.14 |
| S. noctiflora | 40 | 93.6 a | 82 | 93.4 | 1.09 |
| S. pendula | 40 | 90.8 def | 89 | 94.5 | 2.1 |
| S. uniflora | 40 | 90.3 ef | 83 | 94.1 | 2.66 |
| S. viscosa | 40 | 92.6 ab | 83 | 94.5 | 1.33 |
| S. vulgaris | 40 | 91.18 cdef | 83 | 94 | 2.15 |
| S. zawadskii | 40 | 90.02 f | 83 | 94.5 | 2.57 |
| Species | n | J Index (Cardioid) | Min | Max | Standard Dev. |
|---|---|---|---|---|---|
| S. colpophylla | 40 | 85.6 e | 77.7 | 91.1 | 2.96 |
| S. gallica | 40 | 90.0 b | 85.9 | 93.1 | 1.51 |
| S. italica | 40 | 90.1 ab | 85 | 92.7 | 1.74 |
| S. mellifera | 40 | 89.8 bc | 81.2 | 94 | 2.31 |
| S. nutans Chk | 40 | 91.6 a | 83.7 | 95.2 | 2.43 |
| S. nutans Pol | 40 | 91.6 a | 83.8 | 94.9 | 2.46 |
| S. otites | 40 | 90.7 ab | 85.9 | 93.9 | 2.11 |
| S. saxifraga | 40 | 90.3 ab | 85.2 | 94.2 | 1.73 |
| S. schafta | 40 | 82.3 f | 74.9 | 90.3 | 2.88 |
| S. tatarica | 40 | 87.8 d | 81.6 | 90.9 | 1.64 |
| S. wolgensis | 40 | 88.3 cd | 81.8 | 92.2 | 2.28 |
| n | A | P | L | W | AR | C | R | JI M1 | |
|---|---|---|---|---|---|---|---|---|---|
| S. subg Behenantha | 600 | 1.18 a (0.4) | 4.44 a (0.8) | 1.33 a (0.24) | 1.11 a (0.19) | 1.19 a (0.06) | 0.73 a (0.07) | 0.84 a (0.05) | 91.8 a (2.09) |
| S. subg. Silene | 400 | 0.7 b (0.22) | 3.5 b (0.69) | 1.07 b (0.17) | 0.86 b (0.13) | 1.25 b (0.08) | 0.73 a (0.1) | 0.8 b (0.05) | 88.7 b (3.4) |
| J Index Values | S. diclinis (n = 40) | S. latifolia (n = 160) | S.noctiflora (n = 40) |
|---|---|---|---|
| M1 | 90.3 c (2.66) | 92.6 b (1.46) | 93.6 a (1.11) |
| M2 | 91.2 c (2.18) | 93.0 b (1.68) | 94.4 a (1.14) |
| M4 | 91.5 b (2.42) | 92.5 a (1.97) | 89.6 c (2.53) |
| J Index Values | S. conica (n = 40) | S. gallica (n = 40) | S. otites (n = 40) |
|---|---|---|---|
| M1 | 92.1 a (1.29) | 90.0 b (1.52) | 90.7 b (2.13) |
| M3 | 86.2 c (2.22) | 90.4 a (1.01) | 88.8 b (2.48) |
| Species | Lab. | Origin | Annual, Biannual or Perennial |
|---|---|---|---|
| S. acutifolia Link ex Rohrb. | CzR | u (unknown) | P [57] |
| S. colpophylla Wrigley | CzR | France | B,P [58] |
| S. conica L. | CzR | Germany | A [59] |
| S. diclinis (Lag.) M.Laínz | CzR | Pla de Mora (Spain) | P [60] |
| S. dioica (L.) Clairv. | CzR/Pol | Tišnov (CzR)/u | P [29,58] |
| S. gallica L. | CzR | u | AB [29,61] |
| S. italica (L.) Pers. | CzR | u | BP [29,61] |
| S. latifolia Poir. | CzR/Pol (3 seed stocks) | Panenská Rozsíčka (CzR)/Dubidze(Pol) | P [29,56,61] |
| S. mellifera Boiss. and Reut. | Pol | u | BP [62] |
| S. noctiflora L. | CzR | Kuřim (CzR) | A [29,61] |
| S. nutans L. | CzR/Pol | u | P [29,61] |
| S. otites (L.) Wibel | CzR | Rohatec (CzR) | P [58,63] |
| S. pendula L. | CzR | u | A [29] |
| S. saxifraga L. | CzR | u | P [29] |
| S. schafta S.G.Gmel. | CzR | u | P [29] |
| S. tatarica (L.) Pers. | CzR | u | P [29,61] |
| S. uniflora Roth | Pol | u | P [64] |
| S. viscosa (L.) Pers. | CzR | Rohatec (CzR) | BP [61] |
| S. vulgaris (Moench) Garcke | CzR | Lomnička (CzR) | P [29,61,65] |
| S. wolgensis (Hornem.) Otth | CzR | Bashkortostan (RU) | B [29] |
| S. zawadzkii Herbich | CzR | u | P |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Gómez, J.J.; Rewicz, A.; Rodríguez-Lorenzo, J.L.; Janoušek, B.; Cervantes, E. Seed Morphology in Silene Based on Geometric Models. Plants 2020, 9, 1787. https://doi.org/10.3390/plants9121787
Martín-Gómez JJ, Rewicz A, Rodríguez-Lorenzo JL, Janoušek B, Cervantes E. Seed Morphology in Silene Based on Geometric Models. Plants. 2020; 9(12):1787. https://doi.org/10.3390/plants9121787
Chicago/Turabian StyleMartín-Gómez, José Javier, Agnieszka Rewicz, José Luis Rodríguez-Lorenzo, Bohuslav Janoušek, and Emilio Cervantes. 2020. "Seed Morphology in Silene Based on Geometric Models" Plants 9, no. 12: 1787. https://doi.org/10.3390/plants9121787