VS-Cambium-Developer: A New Predictive Model of Cambium Functioning under the Influence of Environmental Factors
Abstract
:1. Introduction
- (1)
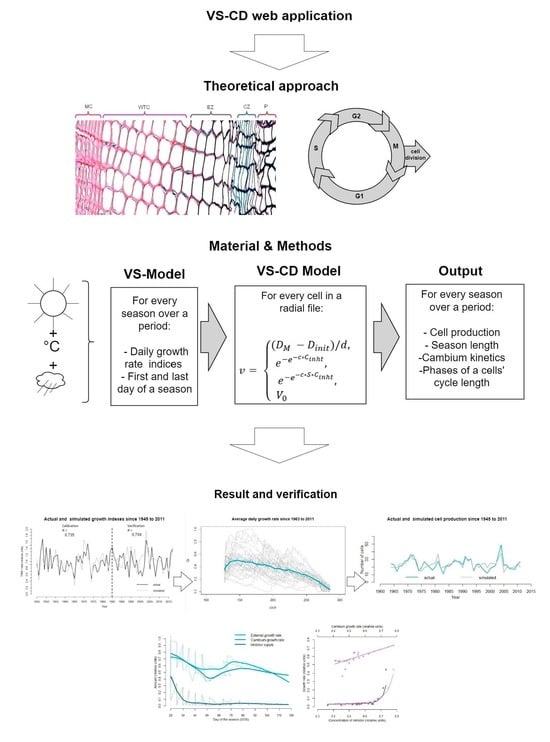
- Presynthetic phase (G1), where the cell increases in size and duplicates its cellular contents;
- (2)
- Synthesis phase (S), which is for DNA coping and synthesis;
- (3)
- Premitotic phase (G2), where the cell increases in size while organelles and proteins prepare for cell division;
- (4)
- Mitosic phase (M), where the cell partitions the two copies of the genetic material of the mother cell into the two daughter cells.
2. Results
- An “average growth season” was simulated for the selected tree sample, and average tree-ring indices and cell production for all available growth seasons were calculated.
- The VS-CD model parameters for the “average growth season” were adjusted to obtain optimal simulation results for the average production and time of initial cell division.
- Parameters constant from season to season were applied to simulate cell production of the selected tree in each season.
- The inhibitor stock for each season was manually calculated from 1963 to 2011, in automatic mode, using a linear regression model.
- (1)
- When cell production data were not prepared (1978 and 2003);
- (2)
- When the significant growth discrepancy between simulated and actual tree-ring indices was observed to exclude incorrect input data in VS-CD model calculations (1977, 1988, 2001, 2008; see Figure S2).
- Verification of the algorithm for the equivalence of the automatically calculated inhibitor supply to the manually calculated inhibitor supply, per season.
- Verification of the algorithm by cell production, estimated with automatically calculated inhibitor supply.
3. Discussion
4. Materials and Methods
4.1. The VS-CD Model: Algorithm for Predicting Cell Production
- The length of the simulated growing season reaches the length of the season calculated by the VS model environmental block outputs;
- The whole supply of the inhibitor is consumed. In this case, the VS-CD model determines the season length.
4.2. Comparison the VS-CD Simulations with Observed Anatomical Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chavchavadze, E.S. Coniferous wood. In Morphological Features, Diagnostic Value; NAUKA Publishing House: Moscow, Russia, 1979. [Google Scholar]
- Larson, P.R. The Vascular Cambium: Development and Structure; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Vaganov, E.A.; Hughes, M.K.; Shashkin, A.V. Growth Dynamics of Conifer Tree Rings: Images of Past and Future Environments; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Wilson, B.F. Mitotic activity in the cambial zone of Pinus strobus. Am. J. Bot. 1966, 53, 364–372. [Google Scholar] [CrossRef]
- Savidge, R.A. Xylogenesis, genetic and environmental regulation—A review. IAWA J. 1996, 17, 269–310. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.K.; Frank, D.; Fonti, P.; Fournier, M. Kinetics of tracheid development explain conifer tree-ring structure. New Phytol. 2014, 203, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Cuny, H.E.; Rathgeber, C.B.K. Xylogenesis: Coniferous trees of temperate forests are listening to the climate tale during the growing season but only remember the last words! Plant Physiol. 2016, 171, 306–317. [Google Scholar] [CrossRef]
- Rossi, S.; Morin, H.; Deslauriers, A.; Plourde, P.Y. Predicting xylem phenology in black spruce under climate warming. Glob. Chang. Biol. 2011, 17, 614–625. [Google Scholar] [CrossRef]
- Nanayakkara, B.; Dickson, A.R.; Meason, D.F. Xylogenesis of Pinus radiata D. Don growing in New Zealand. Ann. For. Sci. 2019, 76, 74. [Google Scholar] [CrossRef]
- Tumajer, J.; Shishov, V.V.; Ilyin, V.A.; Camarero, J.J. Intra-annual growth dynamics of Mediterranean pines and junipers determines their climatic adaptability. Agric. For. Meteorol. 2021, 311, 108685. [Google Scholar] [CrossRef]
- Bhalerao, R.P.; Fischer, U.; Turner, S. Environmental and hormonal control of cambial stem cell dynamics. J. Exp. Bot. 2017, 68, 79–87. [Google Scholar] [CrossRef]
- Buttò, V.; Shishov, V.; Tychkov, I.; Popkova, M.; He, M.; Rossi, S.; Deslauriers, A.; Morin, H. Comparing the cell dynamics of tree-ring formation observed in microcores and as predicted by the Vaganov–Shashkin Model. Front. Plant Sci. 2020, 11, 1268. [Google Scholar] [CrossRef]
- Hacke, U.G.; Lachenbruch, B.; Pittermann, J.; Stefan, M.; Domec, J.C.; Schulte, P.J. The hydraulic architecture of conifers. In Functional and Ecological Xylem Anatomy; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Bossinger, G.; Spokevicius, A.V. Sector analysis reveals patterns of cambium differentiation in poplar stems. J. Exp. Bot. 2018, 69, 4339–4348. [Google Scholar] [CrossRef]
- Uggla, C.; Mellerowicz, E.J.; Sundberg, B. Indole-3-acetic acid controls cambial growth in Scots pine by positional signaling. Plant Physiol. 1998, 117, 113–121. [Google Scholar] [CrossRef]
- Buttò, V.; Rossi, S.; Deslauriers, A.; Morin, H. Is size an issue of time? Relationship between the duration of xylem development and cell traits. Ann. Bot. 2019, 123, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Eric Schaller, G.; Voesenek, L.A.C.J. Focus on ethylene. Plant Physiol. 2015, 169, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Friml, J. Auxin transport—Shaping the plant. Curr. Opin. Plant Biol. 2003, 6, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Immanen, J.; Nieminen, K.; Smolander, O.P.; Kojima, M.; Alonso Serra, J.; Koskinen, P.; Zhang, J.; Elo, A.; Mähönen, A.P.; Street, N.; et al. Cytokinin and auxin display distinct but interconnected distribution and signaling profiles to stimulate cambial activity. Curr. Biol. 2016, 26, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Sang, Y.L.; Cheng, Z.J.; Zhang, X.S. Plant stem cells and de novo organogenesis. New Phytol. 2018, 218, 1334–1339. [Google Scholar] [CrossRef]
- Lin, J.; Frank, M.; Reid, D. No Home without Hormones: How plant hormones control legume nodule organogenesis. Plant Commun. 2020, 1, 100104. [Google Scholar] [CrossRef]
- Uggla, C.; Magel, E.; Moritz, T.; Sundberg, B. Function and dynamics of auxin and carbohydrates during earlywood/latewood transition in Scots pine. Plant Physiol. 2001, 125, 2029–2039. [Google Scholar] [CrossRef]
- Brackmann, K.; Qi, J.; Gebert, M.; Jouannet, V.; Schlamp, T.; Grünwald, K.; Wallner, E.S.; Novikova, D.D.; Levitsky, V.G.; Agustí, J.; et al. Spatial specificity of auxin responses coordinates wood formation. Nat. Commun. 2018, 9, 875. [Google Scholar] [CrossRef]
- Reid, D.; Nadzieja, M.; Novák, O.; Heckmann, A.B.; Sandal, N.; Stougaard, J. Cytokinin biosynthesis promotes cortical cell responses during nodule development. Plant Physiol. 2017, 175, 361–375. [Google Scholar] [CrossRef]
- Tank, J.G.; Thaker, V.S. Cyclin dependent kinases and their role in regulation of plant cell cycle. Biol. Plant. 2011, 55, 201–212. [Google Scholar] [CrossRef]
- Barrera-Rojas, C.H.; Otoni, W.C.; Nogueira, F.T.S. Shaping the root system: The interplay between miRNA regulatory hubs and phytohormones. J. Exp. Bot. 2021, 72, 6822–6835. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.K.; da Montessoro, P.F.; Fusaro, A.F.; Araújo, B.G.; Hemerly, A.S. Plant cdks—Driving the cell cycle through climate change. Plants 2021, 10, 1804. [Google Scholar] [CrossRef] [PubMed]
- Komaki, S.; Sugimoto, K. Control of the plant cell cycle by developmental and environmental cues. Plant Cell Physiol. 2012, 53, 953–964. [Google Scholar] [CrossRef]
- Schrader, J.; Nilsson, J.; Mellerowicz, E.; Berglund, A.; Nilsson, P.; Hertzberg, M.; Sandberg, G. A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem cell identity. Plant Cell 2004, 16, 2278–2292. [Google Scholar] [CrossRef]
- Kang, Y.; Li, W.; Zhang, L.; Qi, L. Over-expression of the cell-cycle gene lacdkb1;2 promotes cell proliferation and the formation of normal cotyledonary embryos during Larix kaempferi somatic embryogenesis. Genes 2021, 12, 1435. [Google Scholar] [CrossRef]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef]
- Li, W.F.; Cui, K.M.; He, X.Q. Regulation of cell cycle regulators by environmental signals during growth-dormancy cycle of trees. Plant Signal. Behav. 2009, 4, 959–961. [Google Scholar] [CrossRef]
- Campbell, L.; Turner, S.; Etchells, P. Regulation of vascular cell division. J. Exp. Bot. 2017, 68, 27–43. [Google Scholar] [CrossRef]
- Li, W.F.; Kang, Y.; Zhang, Y.; Zang, Q.L.; Qi, L.W. Concerted control of the LaRAV1-LaCDKB1;3 module by temperature during dormancy release and reactivation of larch. Tree Physiol. 2021, 41, 1918–1937. [Google Scholar] [CrossRef]
- Deleuze, C.; Houllier, F. Prediction of stem profile of Picea abies using a process-based tree growth model. Tree Physiol. 1995, 15, 113–120. [Google Scholar] [CrossRef]
- Deleuze, C.; Houllier, F. Simple process-based xylem growth model for describing wood microdensitometric profiles. J. Theor. Biol. 1998, 193, 99–113. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.K.; Lebourgeois, F.; Fortin, M.; Fournier, M. Life strategies in intra-annual dynamics of wood formation: Example of three conifer species in a temperate forest in north-east France. Tree Physiol. 2012, 32, 612–625. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.K.; Kiessé, T.S.; Hartmann, F.P.; Barbeito, I.; Fournier, M. Generalized additive models reveal the intrinsic complexity of wood formation dynamics. J. Exp. Bot. 2013, 64, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Morin, H.; Saracino, A.; Motta, R.; Borghetti, M. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 2006, 170, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.M.; Downes, G. A model of stem growth and wood formation in Pinus radiata. Trees Struct. Funct. 2015, 29, 1395–1413. [Google Scholar] [CrossRef]
- Cabon, A.; Fernández-de-Uña, L.; Gea-Izquierdo, G.; Meinzer, F.C.; Woodruff, D.R.; Martínez-Vilalta, J.; De Cáceres, M. Water potential control of turgor-driven tracheid enlargement in Scots pine at its xeric distribution edge. New Phytol. 2020, 225, 209–221. [Google Scholar] [CrossRef]
- Forest, L.; Demongeot, J. Cellular modelling of secondary radial growth in conifer trees: Application to Pinus radiata (D. Don). Bull. Math. Biol. 2006, 68, 753–784. [Google Scholar] [CrossRef]
- Forest, L.; Martín, J.S.; Padilla, F.; Chassat, F.; Giroud, F.; Demongeot, J. Morphogenetic processes: Application to cambial growth dynamics. Acta Biotheor. 2004, 52, 415–438. [Google Scholar] [CrossRef]
- Peters, R.L.; Steppe, K.; Cuny, H.E.; De Pauw, D.J.W.; Frank, D.C.; Schaub, M.; Rathgeber, C.B.K.; Cabon, A.; Fonti, P. Turgor—A limiting factor for radial growth in mature conifers along an elevational gradient. New Phytol. 2021, 229, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, F.P.; Cyrille, C.B.; Fournier, M.; Moulia, B. Modelling wood formation and structure: Power and limits of a morphogenetic gradient in controlling xylem cell proliferation and growth. Ann. For. Sci. 2017, 74, 14. [Google Scholar] [CrossRef]
- Hartmann, F.P.; Rathgeber, C.B.K.; Badel, É.; Fournier, M.; Moulia, B. Modelling the spatial crosstalk between two biochemical signals explains wood formation dynamics and tree-ring structure. J. Exp. Bot. 2021, 72, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Catesson, A.M.; Roland, J.C. Sequential Changes Associated with Cell Wall Formation and Fusion in the Vascular Cambium. IAWA J. 2014, 2, 151–162. [Google Scholar] [CrossRef]
- Drew, D.M.; Pammenter, N.W. Developmental rates and morphological properties of fibres in two eucalypt clones at sites differing in water availability. South. Hemisph. For. J. 2007, 69, 71–79. [Google Scholar] [CrossRef]
- Ridoutt, B.G.; Sands, R. Quantification of the processes of secondary xylem fibre development in Eucalyptus globulus at two height levels. IAWA J. 1994, 15, 417–424. [Google Scholar] [CrossRef]
- Oles, V.; Panchenko, A.; Smertenko, A. Modeling hormonal control of cambium proliferation. PLoS ONE 2017, 12, e0171927. [Google Scholar] [CrossRef]
- Voß, U.; Bishopp, A.; Farcot, E.; Bennett, M.J. Modelling hormonal response and development. Trends Plant Sci. 2014, 19, 311–319. [Google Scholar] [CrossRef]
- Dickinson, M.B.; Jolliff, J.; Bova, A.S. Vascular cambium necrosis in forest fires: Using hyperbolic temperature regimes to estimate parameters of a tissue-response model. Aust. J. Bot. 2004, 52, 757–763. [Google Scholar] [CrossRef]
- Lachaud, S. Some aspects of phytohormonal participation in the control of cambial activity and xylogenesis in tree stems. Ann. Sci. For. 1989, 46, 273s–276s. [Google Scholar] [CrossRef]
- Anchukaitis, K.J.; Evans, M.N.; Hughes, M.K.; Vaganov, E.A. An interpreted language implementation of the Vaganov–Shashkin tree-ring proxy system model. Dendrochronologia 2020, 60, 125677. [Google Scholar] [CrossRef]
- Shishov, V.V.; Tychkov, I.I.; Anchukaitis, K.J.; Zelenov, G.K.; Vaganov, E.A. A band model of cambium development: Opportunities and prospects. Forests 2021, 12, 1361. [Google Scholar] [CrossRef]
- Belousova, D.A.; Shishov, V.V.; Babushkina, E.A.; Vaganov, E.A. VS-Cambium-Developer: A new approach to modeling the functioning of the cambial zone of conifers under the influence of environmental factors. Russ. J. Ecol. 2021, 52, 358–367. [Google Scholar] [CrossRef]
- Savidge, R.A. The tracheid-differentiation factor of conifer needles. Int. J. Plant Sci. 1994, 155, 272–290. [Google Scholar] [CrossRef]
- Fonti, M.V.; Tychkov, I.I.; Shishov, V.V.; Shashkin, A.V.; Prokushkin, A.S. Plant-soil-climate interaction in observed and simulated tree-radial growth dynamics of downy birch in permafrost. Front. Plant Sci. 2022, 13, 780153. [Google Scholar] [CrossRef]
- Kang, J.; Shishov, V.V.; Tychkov, I.; Zhou, P.; Jiang, S.; Ilyin, V.A.; Ding, X.; Huang, J.G. Response of model-based cambium phenology and climatic factors to tree growth in the Altai Mountains, Central Asia. Ecol. Indic. 2022, 143, 109393. [Google Scholar] [CrossRef]
- Arzac, A.; Tychkov, I.; Rubtsov, A.; Tabakova, M.A.; Brezhnev, R.; Koshurnikova, N.; Knorre, A.; Büntgen, U. Phenological shifts compensate warming-induced drought stress in southern Siberian Scots pines. Eur. J. For. Res. 2021, 140, 1487–1498. [Google Scholar] [CrossRef]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The yin-yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef]
- Su, Y.H.; Liu, Y.B.; Zhang, X.S. Auxin-cytokinin interaction regulates meristem development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef]
- Fischer, U.; Kucukoglu, M.; Helariutta, Y.; Bhalerao, R.P. The Dynamics of Cambial Stem Cell Activity. Annu. Rev. Plant Biol. 2019, 70, 293–319. [Google Scholar] [CrossRef]
- Valeriano, C.; Gutiérrez, E.; Colangelo, M.; Gazol, A.; Sánchez-Salguero, R.; Tumajer, J.; Shishov, V.; Bonet, J.A.; Martínez de Aragón, J.; Ibáñez, R.; et al. Seasonal precipitation and continentality drive bimodal growth in Mediterranean forests. Dendrochronologia 2023, 78, 126057. [Google Scholar] [CrossRef]
- Valeriano, C.; Gazol, A.; Colangelo, M.; González de Andrés, E.; Camarero, J.J. Modeling climate impacts on tree growth to assess tree vulnerability to drought during forest dieback. Front. Plant Sci. 2021, 12, 672855. [Google Scholar] [CrossRef]
- Shishov, V.V.; Tychkov, I.I.; Popkova, M.I.; Ilyin, V.A.; Bryukhanova, M.V.; Kirdyanov, A.V. VS-oscilloscope: A new tool to parameterize tree radial growth based on climate conditions. Dendrochronologia 2016, 39, 42–50. [Google Scholar] [CrossRef]
- Shah, S.K.; Touchan, R.; Babushkina, E.; Shishov, V.V.; Meko, D.M.; Abramenko, O.V.; Belokopytova, L.V.; Hordo, M.; Jevšenak, J.; Kędziora, W.; et al. August to july precipitation from tree rings in the forest-steppe zone of Central Siberia (Russia). Tree Ring Res. 2015, 71, 37–44. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Čufar, K.; Cuny, H.E.; Deslauriers, A.; Fonti, P.; Frank, D.; Gričar, J.; Gruber, A.; Huang, J.G.; et al. Pattern of xylem phenology in conifers of cold ecosystems at the Northern Hemisphere. Glob. Chang. Biol. 2016, 22, 3804–3813. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Deslauriers, A.; Morin, H. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia 2003, 21, 33–39. [Google Scholar] [CrossRef]
- Giorgi, F.M.; Ceraolo, C.; Mercatelli, D. The R Language: An Engine for Bioinformatics and Data Science. Life 2022, 12, 648. [Google Scholar] [CrossRef] [PubMed]
- Crawley, M.J. The R Book; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Rinne, K.T.; Saurer, M.; Kirdyanov, A.V.; Bryukhanova, M.V.; Prokushkin, A.S.; Churakova Sidorova, O.V.; Siegwolf, R.T.W. Examining the response of needle carbohydrates from Siberian larch trees to climate using compound-specific δ13C and concentration analyses. Plant Cell Environ. 2015, 38, 2340–2352. [Google Scholar] [CrossRef]
- Cook, E.R.; Kairiukstis, L.A. (Eds.) Methods of Dendrochronology: Applications in the Environmental Sciences; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Holmes, R. Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull. 1983, 43, 51–67. [Google Scholar]
- Grissino-Mayer, H.D. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree Ring Res. 2001, 57, 205–221. [Google Scholar]
- Cook, E.R.; Holmes, R.L. Guide for computer program ARSTAN. Int. Tree Ring Data Bank Program Libr. Version 1996, 2, 75–87. [Google Scholar]
- Cook, E.R.; Krusic, P.J. Program ARSTAN: A Tree-Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling, with Interactive Graphics; Lamont-Doherty Earth Observatory, Columbia University: Palisades, NY, USA, 2005. [Google Scholar]
- Silkin, P.P. Methods of Multiparameter Analysis of Conifers Tree-Rings Structure; Siberian Federal University: Krasnoyarsk, Russia, 2010. [Google Scholar]








| Parameters | Dimension | Value |
|---|---|---|
| Critical cell diameter transitioning to phase S from phase G1 | μm | 8.5 |
| Critical cell diameter transitioning to phase G2 from phase S | μm | 8.7 |
| Critical cell diameter transitioning to phase M from phase G2 | μm | 9.0 |
| Critical cell diameter for division | μm | 9.5 |
| Minimum inhibitor concentration required for cell division | relative units | 0.03 |
| The growth rate V0 in S, G2, M phases | μm/day | 0.68 |
| Parameter of growth rate in phase G1 | relative units | 0.071 |
| Diameter of initial cell | μm | 2.0 |
| Stress coefficient of growth rate in phase G1 | relative units | 100 |
| Coefficient of inhibitor distribution between daughter cells | relative units | 0.8286 |
| N | Type | Parameters | Dimension | Description |
|---|---|---|---|---|
| 1 | Parameters and data calculated by the environmental block of the VS model (see [55,67] for more details) | Length of the growing season | day | Estimated by VS model and depends only on external climatic factors |
| 2 | Daily integral growth rates | relative units | Computed by VS model from daily data of temperature, precipitation and hours of sunlight | |
| 3 | Start of the growing season | day | Determined when a specified sum of temperature is reached for a period | |
| 4 | End of the growing season | day | Determined when the integral growth rate reaches a critical value | |
| 5 | Fixed VS-CD model parameters for all seasons | Critical cell diameter transitioning to phase S from phase G1 | μm | The positive approximate value is manually set using actual anatomical measurements |
| 6 | Critical cell diameter transitioning to phase G2 from phase S | μm | ||
| 7 | Critical cell diameter transitioning to phase M from phase G2 | μm | ||
| 8 | Critical cell diameter for division | μm | ||
| 9 | Minimum inhibitor concentration required for cell division | relative units | The positive approximate value is manually set | |
| 10 | The growth rate V0 in S, G2, M phases | μm/day | The approximate value is manually set from 0 to 1 using VS model growth rates | |
| 11 | Parameter of growth rate in phase G1 | relative units | The approximate value is manually set (see “c” in Equation (3) below) | |
| 12 | Initial cell diameter | μm | The approximate value is manually set using actual anatomical measurements | |
| 13 | Stress coefficient of growth rate in phase G1 | relative units | The approximate value is manually set (see “S” in Equation (3) below) | |
| 14 | Coefficient of inhibitor distribution between daughter cells | relative units | The approximate value is manually set from 0.01 to 0.99 | |
| 15 | VS-CD model parameters representing the cell growth rate | Supply of the inhibitor in the initial cell at the start of the growing season | relative units | Calculated automatically (see Equation (1) below) |
| Parameters | Dimension |
|---|---|
| Date of current cell appearance in the season | Days (accuracy 0.001) |
| Timing of current cell phase G1 | Days (accuracy 0.001) |
| Timing of current cell phase S | Days (accuracy 0.001) |
| Timing of current cell phase G2 | Days (accuracy 0.001) |
| Timing of current cell phase M | Days (accuracy 0.001) |
| Timing of current cell cycle | Days (accuracy 0.001) |
| Amount of inhibitor received from mother cell | relative units |
| Current cell growth rate in phase G1 | μm/day |
| Inhibitor concentration in M phase (needed to assess whether further cell division is possible) | relative units |
| Unique cell ID | text string |
| Mother cell ID | text string |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belousova, D.A.; Shishov, V.V.; Arzac, A.; Popkova, M.I.; Babushkina, E.A.; Huang, J.-G.; Yang, B.; Vaganov, E.A. VS-Cambium-Developer: A New Predictive Model of Cambium Functioning under the Influence of Environmental Factors. Plants 2023, 12, 3594. https://doi.org/10.3390/plants12203594
Belousova DA, Shishov VV, Arzac A, Popkova MI, Babushkina EA, Huang J-G, Yang B, Vaganov EA. VS-Cambium-Developer: A New Predictive Model of Cambium Functioning under the Influence of Environmental Factors. Plants. 2023; 12(20):3594. https://doi.org/10.3390/plants12203594
Chicago/Turabian StyleBelousova, Daria A., Vladimir V. Shishov, Alberto Arzac, Margarita I. Popkova, Elena A. Babushkina, Jian-Guo Huang, Bao Yang, and Eugene A. Vaganov. 2023. "VS-Cambium-Developer: A New Predictive Model of Cambium Functioning under the Influence of Environmental Factors" Plants 12, no. 20: 3594. https://doi.org/10.3390/plants12203594