Micromorphology and Chemical Studies on Anacardium occidentale L. Stem Bark as an Herbal Medicine
Abstract
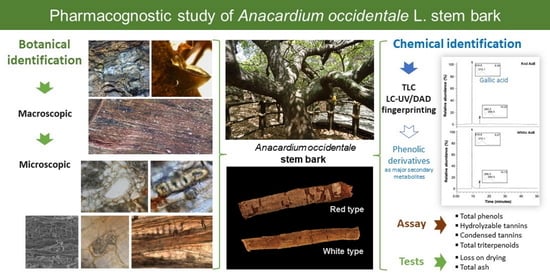
:1. Introduction
2. Results
2.1. Macroscopy
2.2. Microscopy
2.3. Powder
2.4. Histochemical Tests
2.5. Chemical Studies
2.5.1. Drug-Extract Ratio of Aqueous Extracts
2.5.2. Thin-Layer Chromatography
2.5.3. LC-UV Analysis
2.5.4. Quantification of Secondary Metabolites by Spectrophotometry
2.6. Tests
2.6.1. Loss on Drying
2.6.2. Total Ash
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Macroscopic Analysis
4.4. Light Microscopy
4.5. Scanning Electron Microscopy
4.6. Histochemical Tests
4.7. Quantitative Analysis
4.8. Chemical Studies
4.8.1. Extract Preparation
4.8.2. Thin-Layer Chromatography
4.8.3. LC-UV/DAD Analysis
4.8.4. Quantification of Secondary Metabolites by Spectrophotometry
- Total phenolic content
- Hydrolyzable Tannin Content
- Condensed Tannin Content
- Total Triterpenoid Content
4.9. Tests
4.9.1. Loss on Drying
4.9.2. Total Ash
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, B.; Gültekin-Özgüven, M.; Kirkin, C.; Özçelik, B.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; da Silva, T.G.; Coutinho, H.D.M.; Amina, B.; et al. Antioxidant, Antimicrobial, and Anticancer Effects of Anacardium Plants: An Ethnopharmacological Perspective. Front. Endocrinol. 2020, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- The World Flora Online. Anacardium occidentale L. Available online: http://www.worldfloraonline.org/taxon/wfo-0000533072#L (accessed on 1 October 2022).
- Diniz, M.A.; Martins, S.; Gomes, E.; Silva, O. Contribuição Para o Conhecimento de Plantas Medicinais Da Guiné-Bissau. Port. Acta Biol. 2000, 19, 417–427. [Google Scholar]
- de Erédia, M. Suma de Árvores e Plantas Da Índia Intra Ganges, 1st ed.; Comissão Nacional para as Comemorações dos Descobrimentos Portugueses: Lisbon, Portugal, 2001; pp. 109–115. [Google Scholar]
- Ferrão, J. A Aventura das Plantas e Os Descobrimentos Portugueses; Chaves Ferreira Publicações: Lisbon, Portugal, 2005; pp. 93–99. [Google Scholar]
- Hutchinson, J.; Dalziel, J.; Keay, R.; Hepper, F. Flora of West Tropical Africa, 2nd ed.; Crown Agents for Overseas Governments and Administrations: London, UK, 1952; Volume I, pp. 951–952. [Google Scholar]
- Catarino, L.; Menezes, Y.; Sardinha, R. Cashew Cultivation in Guinea-Bissau—Risks and Challenges of the Success of a Cash Crop. Sci. Agric. 2015, 72, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, F.; Catarino, L.; Batista, D.; Indjai, B.; Duarte, M.C.; Romeiras, M.M. Cashew as a High Agricultural Commodity in West Africa: Insights towards Sustainable Production in Guinea-Bissau. Sustainability 2017, 9, 1666. [Google Scholar] [CrossRef] [Green Version]
- Antão, L.A.R. Anacardium occidentale Linn–Estudo Farmacognóstico da Casca e Alguns Ensaios Sobre o Óleo. Garcia Orta 1959, 7, 551–567. [Google Scholar]
- Azam-Ali, S.; Judge, E. Small-Scale Cashew Nut Processing; FAO: Rome, Italy, 2001; pp. 1–70. [Google Scholar]
- Lim, T.K. Anacardium occidentale. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; Volume 1, pp. 45–68. [Google Scholar]
- Indjai, B.; Catarino, L.; Mourão, D. Mezinhos de Orango—Plantas Medicinais e Pessoas da Ilha da Rainha Pampa; Instituto da Biodiversidade e das Áreas Protegidas: Bissau, Guinea-Bissau, 2010; p. 60. [Google Scholar]
- Chabi Sika, K.; Ahoton, L.E.; Adebo, I.; Adigoun, F.A.; Saidou, A.; Kotchoni, S.O.; Ahanchede, A. Indigenous Knowledge and Traditional Management of Cashew (Anacardium occidentale L.) Genetic Resources in Benin. J. Exp. Biol. Agric. Sci. 2013, 1, 375–382. [Google Scholar]
- Mitra, R.; Mitchell, B.; Gray, C.; Orbell, J.; Coulepis, T.; Muralitharan, M. Medicinal Plants of Brazil. Asia Pacific Biotech News, 15 June 2007; Volume 11, 689–706. [Google Scholar]
- Omolaso, B.O.; Oluwole, F.S.; Odukanmi, O.A.; Adesanwo, J.K.; Ishola, A.A.; Adewole, K.E. Evaluation of the Gastrointestinal Anti-Motility Effect of Anacardium occidentale Stem Bark Extract: A Mechanistic Study of Antidiarrheal Activity. J. Pharm. Anal. 2021, 11, 776–782. [Google Scholar] [CrossRef]
- Coutinho, H.; Barbosa-Filho, V.; Waczuk, E.; Leite, N.; Costa, J.G.; Lacerda, S.; Adedara, I.; Kamdem, J.P.; Posser, T.; Menezes, I. Phytocompounds and Modulatory Effects of Anacardium microcarpum (Cajui) on Antibiotic Drugs Used in Clinical Infections. Drug Des. Dev. Ther. 2015, 9, 5965–5972. [Google Scholar] [CrossRef] [Green Version]
- Encarnação, S.; de Mello-Sampayo, C.; Graça, N.A.G.; Catarino, L.; da Silva, I.B.M.; Lima, B.S.; Silva, O.M.D. Total Phenolic Content, Antioxidant Activity and Pre-Clinical Safety Evaluation of an Anacardium occidentale Stem Bark Portuguese Hypoglycemic Traditional Herbal Preparation. Ind. Crops Prod. 2016, 82, 171–178. [Google Scholar] [CrossRef]
- Ojezele, M.O.; Agunbiade, S. Phytochemical Constituents and Medicinal Properties of Different Extracts of Anacardium occidentale and Psidium guajava. Asian J. Biomed. Pharm. 2013, 3, 20–23. [Google Scholar]
- Eliakim-Ikechukwu, C.; Obri, A.; Akpa, O. Phytochemical and Micronutrient Composition of Anacardium occidentale Linn (Cashew) Stem-Bark Hydroethanolic Extract and Its Effect on the Fasting Blood Glucose Levels and Body Weight of Diabetic Wistar Rats. Internet J. Nutr. Wellness 2009, 10, 1–6. [Google Scholar]
- Chaves, M.H.; das Graças Lopes, C.A.M.; Lopes, J.A.D.; da Costa, D.A.; de Oliveira, C.A.A.; e Francisco, A.F.C.; Brito Júnior, E.M. Fenóis Totais, Atividade Antioxidante e Constituintes Químicos de Extratos de Anacardium occidentale L., Anacardiaceae. Rev. Bras. Farm. 2010, 20, 106–112. [Google Scholar] [CrossRef]
- Shehu, A.; Ponnapalli, M.G.; Mahboob, M.; Prabhakar, P.V.; Olatunji, G.A. New N-Nonadecanoyl-β-Sitosterol and Other Constituents from the Stem-Bark of Anacardium occidentale. Nat. Prod. Res. 2021, 35, 1357–1363. [Google Scholar] [CrossRef]
- Vilar, M.; de Souza, G.; Vilar, D.; Leite, J.; Raffin, F.; Barbosa-Filho, J.; Nogueira, F.; Rodrigues-Mascarenhas, S.; Moura, T. Assessment of Phenolic Compounds and Anti-Inflammatory Activity of Ethyl Acetate Phase of Anacardium occidentale L. Bark. Molecules 2016, 21, 1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander-Lindo, R.L.; Morrison, E.Y.S.A.; Nair, M.G.; McGrowder, D.A. Effect of the Fractions of the Hexane Bark Extract and Stigmast-4-En-3-One Isolated from Anacardium occidentale on Blood Glucose Tolerance Test in an Animal Model. Int. J. Pharmacol. 2007, 3, 41–47. [Google Scholar] [CrossRef]
- Encarnação, S.; Mello-Sampayo, C.; Lima, B.; Silva, O. Evaluation of the Safety of a Traditional Herbal Formulation with Anacardium occidentale Bark. Planta Med. 2012, 78, 1229. [Google Scholar] [CrossRef]
- Encarnação, S.; Malmir, M.; Sousa, D.; da Silva, I.; Mello-Sampayo, C.; Serrano, R.; Lima, B.; Silva, O. Phenol Content, Antioxidant and α- and β-Glucosidase Inhibitory Activities of an Anacardium occidentale Stem Bark Traditional Herbal Preparation. Planta Med. 2014, 80, 1496. [Google Scholar] [CrossRef]
- Encarnação, S.; de Mello-Sampayo, C.; Carrapiço, B.; São Braz, B.; Jordão, A.P.; Peleteiro, C.; Catarino, L.; Moreira Da Silva, I.B.; Gouveia, L.F.; Lima, B.S.; et al. Anacardium occidentale Bark as an Antidiabetic Agent. Plants 2022, 11, 2637. [Google Scholar] [CrossRef]
- Robinson, M.M.; Zhang, X. The World Medicines Situation 2011—Traditional Medicines: Global Situation, Issues and Challenges; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Kasilo, O.M.J.; Wambebe, C. Traditional and Complementary Medicine in Global Health Care. In Handbook of Global Health; Haring, R., Kickbusch, I., Ganten, D., Moeti, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–47. [Google Scholar]
- World Health Organization. WHO Traditional Medicine Strategy 2014–2023. Available online: https://www.who.int/publications/i/item/9789241506096 (accessed on 1 September 2022).
- Petrovska, B.B. Historical Review of Medicinal Plants’ Usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Guidelines on Safety Monitoring of Herbal Medicines in Pharmacovigilance Systems; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- World Health Organization. Quality Control Methods for Herbal Materials. Available online: https://apps.who.int/iris/handle/10665/44479 (accessed on 1 October 2022).
- Upton, R.; Graff, A.; Jolliffe, G.; Länger, R.; Williamson, E. American Herbal Pharmacopoeia: Botanical Pharmacognosy—Microscopic Characterization of Botanical Medicines; Upton, R., Graff, A., Jolliffe, G., Länger, R., Williamson, E., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 240–242. [Google Scholar]
- Gimenez, A.M.; Moglia, G. Estructura Cortical de Anardiaceas Argentinas. For. Syst. 1995, 4, 189–203. [Google Scholar]
- Madjitoloum, B.S.; Talla, E.; Nyemb, J.N.; Ngassoum, M.B.; Tsatsop, T.R.K.; Mahmout, Y. Comparative Survey of Three Processes Used for the Extraction of Total Phenol Content and Total Flavonoid Content of Anacardium occidentale L. and the Assessment of Its Antioxidant Activity. Afr. J. Biotechnol. 2018, 17, 1265–1273. [Google Scholar] [CrossRef] [Green Version]
- European Directorate for the Quality of Medicines and Healthcare. Frangula Bark. In European Pharmacopoeia 11.0; Council of Europe: Strasbourg, France, 2022; p. 1516. [Google Scholar]
- European Directorate for the Quality of Medicines and Healthcare. Herbal Drugs: Sampling and Sample Preparation. In European Pharmacopoeia 11.0; Council of Europe: Strasbourg, France, 2022; p. 329. [Google Scholar]
- European Directorate for the Quality of Medicines and Healthcare. Herbal Drugs: Sieves. In European Pharmacopoeia 11.0; Council of Europe: Strasbourg, France, 2022; p. 18. [Google Scholar]
- European Directorate for the Quality of Medicines and Healthcare. Loss on Drying. In European Pharmacopoeia 11.0; Council of Europe: Strasbourg, France, 2022; p. 63. [Google Scholar]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 359–364. [Google Scholar]
- Hemingway, R.W.; Karchesy, J.J. Chemistry and Significance of Condensed Tannins; Plenum Press: New York, NY, USA, 1989; pp. 141–144. [Google Scholar]
- Silva, O.M.D. Estudo Etnofarmacológico de Espécies Da Flora Da Guiné-Bissau Com Actividade Antimicrobiana. Ph.D. Thesis, Faculty of Pharmacy, Universidade de Lisboa, Lisbon, Portugal, 2004; pp. 275–276. [Google Scholar]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in Wood: Comparison of Different Estimation Methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Willis, R.B.; Allen, P.R. Improved Method for Measuring Hydrolyzable Tannins Using Potassium Iodate. Analyst 1998, 123, 435–439. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The Conversion of Procyanidins and Prodelphinidins to Cyanidin and Delphinidin. Phytochemistry 1986, 25, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.L.; Lin, C.S.; Lai, G.H. Phytochemical Characteristics, Free Radical Scavenging Activities, and Neuroprotection of Five Medicinal Plant Extracts. Evid-Based Complement. Altern. Med. 2012, 2012, 984295. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines and Healthcare. Total Ash. In European Pharmacopoeia 11.0; Council of Europe: Strasbourg, France, 2022; p. 153. [Google Scholar]





| Dimension (cm) | Red AoB | White AoB | ||||
|---|---|---|---|---|---|---|
| Min a–Max b | Mean | SEM c | Min–Max | Mean | SEM | |
| Length | 21.0–32.5 | 26.2 | 2.4 | 21.3–37.2 | 28.0 | 4.8 |
| Width | 2.2–4.8 | 3.3 | 0.6 | 2.4–7.0 | 4.5 | 1.3 |
| Thickness | 0.1–0.2 | 0.1 | 0.0 | 0.1–0.5 | 0.3 | 0.1 |
| Anatomical Characteristic | Red AoB (μm2) | White AoB (μm2) | ||||
|---|---|---|---|---|---|---|
| Min a–Max b | Mean | SEM c | Min–Max | Mean | SEM | |
| Area of phellem cells | 266.68–1771.56 | 994.93 | 51.65 | 71.81–11679.06 | 1224.61 | 464.35 |
| Area of parenchyma cells | 89.20–2172.55 | 915.46 | 66.23 | 8.16–12746.67 | 1208.61 | 195.39 |
| Area of sclereid cells with thick cell walls | 84.84–3672.37 | 894.44 | 101.54 | 20.24–2433.64 | 707.71 | 83.34 |
| Area of sclereid cells with thin cell walls | 62.73–3041.63 | 1370.51 * | 100.89 | 5.75–4350.91 | 955.27 | 117.21 |
| Area of secretory ducts | 984.14–21934.02 | 7076.19 | 1145.72 | 90.96–29925.98 | 7957.99 | 1260.12 |
| Area of medullary rays’ cells | 2.19–2688.02 | 609.67 | 55.63 | 17.22–2820.15 | 664.51 | 65.41 |
| Area of the calcium oxalate druses | 13.31–629.41 | 279.32 * | 34.04 | 55.78–7303.00 | 575.17 | 102.03 |
| Area of the starch grains | 0.65–44.35 | 9.43 * | 1.54 | 1.88–191.92 | 18.25 | 2.73 |
| Compounds | Red AoB | White AoB | Coloration | Distribution |
|---|---|---|---|---|
| Alkaloids | - | - | ----- | ----- |
| Phenols | + | + | Reddish-purple | Major compounds on cortical parenchyma |
| Starch | + | + | Violet | Mainly in parenchymatous cells |
| Tannins | + | + | Blue and brownish colorations | Heterogeneous distribution |
| Triterpenoids | + | + | Reddish-purple | Mainly in thick-walled sclereids |
| Content Assay | AoB Extract | |
|---|---|---|
| Red Type | White Type | |
| Condensed Tannins (mg CAE/g AoB) | 143.69 ± 4.67 * | 73.79 ± 4.46 |
| Hydrolyzable Tannins (mg GAE/g AoB) | 9.38 ± 1.65 * | 20.50 ± 1.00 |
| Total Phenols (mg GAE/g AoB) | 31.39 ± 0.50 | 31.36 ± 0.54 |
| Total Triterpenoids (mg OAE/g AoB) | 49.18 ± 0.82 * | 62.18 ± 1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encarnação, S.; Serrano, R.; Almeida, C.; Silva, O. Micromorphology and Chemical Studies on Anacardium occidentale L. Stem Bark as an Herbal Medicine. Plants 2023, 12, 7. https://doi.org/10.3390/plants12010007
Encarnação S, Serrano R, Almeida C, Silva O. Micromorphology and Chemical Studies on Anacardium occidentale L. Stem Bark as an Herbal Medicine. Plants. 2023; 12(1):7. https://doi.org/10.3390/plants12010007
Chicago/Turabian StyleEncarnação, Sofia, Rita Serrano, Cristina Almeida, and Olga Silva. 2023. "Micromorphology and Chemical Studies on Anacardium occidentale L. Stem Bark as an Herbal Medicine" Plants 12, no. 1: 7. https://doi.org/10.3390/plants12010007