Screening and Evaluation for Antixenosis Resistance in Wheat Accessions and Varieties to Grain Aphid, Sitobion miscanthi (Takahashi) (Hemiptera: Aphididae)
Abstract
:1. Introduction
2. Results
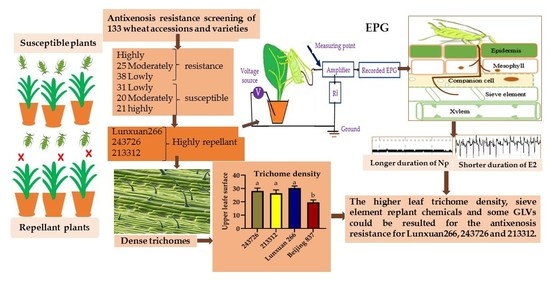
2.1. Preliminary Antixenosis Resistance Screening
2.2. Choice Test for Further Antixenosis Identification
2.3. Measuring Sitobion miscanthi Feeding Behavior by EPG
2.4. Trichome Density
2.5. Correlation Analysis between Number of Winged Sitobion micanthi Settling and EPG Parameters on Wheat Seedlings
2.6. Correlation Analysis between Number of Aphids Settling and Trichome Density on Wheat Accessions and Varieties
3. Discussion
3.1. Preliminary Antixenosis Resistance Screening
3.2. Aphid Bioassays for Further Antixenosis Resistance Tests
3.3. Electrical Penetration Graph
3.4. Trichome Density
4. Materials and Methods
4.1. Insects and Plants
4.2. Preliminary Antixenosis Resistance Screening of Wheat Accession and Varieties against Sitobion miscanthi
4.3. Aphid Bioassay for Further Antixenosis Resistance Test (Choice Test)
4.4. Feeding Trial with EPG Recording
4.5. Trichomes Density
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, S.; Niaz, U. Effect of aphid species infestation on wheat crop, Triticum aestivum L. and its yield. J. Agric. Sci. Bot. 2019, 3, 17–20. [Google Scholar]
- Rehman, A.; Jingdong, L. An econometric analysis of major Chinese food crops: An empirical study. Cogent Econ. Financ. 2017, 122, 1323372. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Xin, W.; Wei, Y.; Zhang, J.; Guo, L. Wheat breeding in northern China: Achievements and technical advances. Crop J. 2019, 7, 718–729. [Google Scholar] [CrossRef]
- Anteneh, A.; Asrat, D. Wheat production and marketing in Ethiopia. Cogent Food Agric. 2020, 6, 1778893. [Google Scholar] [CrossRef]
- USDA. Ethiopia: Grain and Feed Annual. 2020; pp. 1–14. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Grain%20and%20Feed%20Annual_Addis%20Ababa_Ethiopia_03-15-2020. (accessed on 3 April 2020).
- Tadesse, W.; Bishaw, Z.; Assefa, S. Wheat production and breeding in Sub-Saharan Africa climate change. Int. J. Clim. Chang. Strateg. Manag. 2018, 11, 696–715. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Pan, S.; Fan, M.; Chu, B.; Ma, Z.; Gao, F.; Zhao, Z. Cultivar mixture enhances crop yield by decreasing aphids. Agronomy 2022, 12, 335. [Google Scholar] [CrossRef]
- Shavit, R.; Batyrshina, Z.; Dotan, N.; Tzin, V. Cereal aphids differently affect benzoxazinoid levels in durum wheat. PLoS ONE 2018, 13, e0208103. [Google Scholar] [CrossRef]
- Liu, X.F.; Hu, X.S.; Keller, M.A.; Zhao, H.Y.; Wu, Y.F.; Liu, T.X. Tripartite interactions of barley yellow dwarf virus, Sitobion avenae and wheat varieties. PLoS ONE 2014, 9, e106639. [Google Scholar] [CrossRef] [Green Version]
- Jaouannet, M.; Rodriguez, P.A.; Thorpe, P.; Lenoir, G.C.J.; MacLeod, R.B.; Escudero-Martinez, C. Plant immunity in plant-aphid interactions. Front. Plant Sci. 2014, 5, 663. [Google Scholar] [CrossRef] [Green Version]
- Bel, A.J.E.; Will, T. Functional evaluation of proteins in watery and gel saliva of aphids. Front. Plant Sci. 2016, 7, 1840. [Google Scholar]
- Cabrera-Brandt, M.A.; Verdugo, J.A.; Ramirez, C.C.; Lacroze, J.P.; Sauge, M.H.; Figueroa, C.C. Intra-specific variation of behavioral signals in suppressing plant defenses in the green peach aphid Myzus persicae, feeding on the resistant wild peach Prunus davidiana. J. Pest Sci. 2015, 88, 259–266. [Google Scholar] [CrossRef]
- Kaur, H.; Salh, P.K.; Singh, B. Role of defense enzymes and phenolics in resistance of wheat crop (Triticum aestivum L.) towards aphid complex. J. Plant Interact. 2017, 12, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Aradottir, G.I.; Martin, J.L.; Clark, S.J.; Pickett, J.A.; Smart, L.E. Searching for wheat resistance to aphids and wheat bulb fly in the historical Watkins and Gediflux wheat collections. Ann. Appl. Biol. 2017, 170, 179–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- War, A.R.; Taggar, G.K.; Hussain, B.; Taggar, M.S.; Nair, R.M.; Sharma, H.C. Plant defense against herbivory and insect adaptations. AoB Plants 2018, 10, ply037. [Google Scholar]
- Botha, A.M.; van Eck, L.; Burger, N.F.V.; Swanevelder, Z.H. Near-isogenic lines of Triticum aestivum with distinct modes of resistance exhibit dissimilar transcriptional regulation during Diuraphis noxia feeding. Biol. Open 2014, 3, 1116–1126. [Google Scholar] [CrossRef] [Green Version]
- Marimuthu, M.; Smith, C.M.; Marimuthu, M.; Smith, C.M. Barley tolerance of Russian wheat aphid involves expression of defense response and developmental genes Barley tolerance of Russian wheat aphid (Hemiptera: Aphididae) biotype 2 herbivory involves expression of defense response and developmental genes. Plant Signal. Behav. 2012, 2324, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Peterson, R.K.D.; Varella, A.C.; Higley, L.G. Tolerance: The forgotten child of plant resistance. Peer J. 2017, 5, e3934. [Google Scholar] [CrossRef]
- Zheng, S.J.; Dicke, M. Ecological genomics of plant-insect interactions: From gene to community. Plant Physiol. 2008, 146, 812–817. [Google Scholar] [CrossRef] [Green Version]
- Skoczek, A.; Piesik, D.; Wenda-Piesik, A.; Buszewski, B.; Bocianowski, J.; Wawrzyniak, M. Volatile organic compounds released by maize following herbivory or insect extract application and communication between plants. J. Appl. Entomol. 2017, 141, 630–643. [Google Scholar] [CrossRef]
- War, A.; Paulraj, M.; Ahmad, T.; Buhroo, T.; Hussain, B.; Ignacimuthu, S.; Sharm, H. Mechanisms of plant defense against insect herbivores mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 2324, 1306–1320. [Google Scholar] [CrossRef] [Green Version]
- Bahlmann, L.; Govender, P.; Botha, A. Leaf epicuticular wax ultrastructure and trichome presence on Russian wheat aphid (Diuraphis noxia) resistant and susceptible leaves. Afr. Entomol. 2003, 11, 59–64. [Google Scholar]
- Becker, C.; Desneux, N.; Monticelli, L.; Fernandez, X.; Michel, T.; Lavoir, A. Effects of abiotic factors on HIPV-mediated interactions between plants and parasitoids. BioMed Res. Int. 2015, 2015, 342982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenda-Piesik, A. Volatile organic compound emissions by winter wheat plants (Triticum aestivum L.) under Fusarium spp. infestation and various abiotic conditions. Pol. J. Environ. Stud. 2011, 20, 1335–1342. [Google Scholar]
- Cheng, A.; Lou, Y.; Mao, Y.; Lu, S.; Wang, L.; Chen, X. Plant terpenoids: Biosynthesis and ecological functions. J. Integr. Plant Biol. 2007, 49, 179–186. [Google Scholar] [CrossRef]
- Balmer, D.; Flors, V.; Glauser, G.; Mauch-Mani, B. Metabolomics of cereals under biotic stress: Current knowledge and techniques. Front. Plant Sci. 2013, 4, 82. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.B.; Chen, J.L.; Yong, L.I.U.; Francis, F.; Haubruge, E.; Bragard, C.; Sun, J.-R. Influence of garlic intercropping or active emitted volatiles in releasers on aphid and related beneficial in wheat fields in China. J. Integr. Agric. 2013, 12, 467–473. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, L.; Liu, Y.; Chen, J.; Francis, F. Use of slow-release plant infochemicals to control aphids: A first investigation in a Belgian wheat field. Sci. Rep. 2016, 6, 31552. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, D.G.; Brisson, J.A. Aphids: A model for polyphenism and epigenetics. Genet. Res. Int. 2012, 2012, 431531. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.H.; Chen, J.L.; Cheng, D.F.; Sun, J.R.; Liu, Y.; Francis, F. Discovery of English grain aphid (Hemiptera: Aphididae) biotypes in China. J. Econ. Entomol. 2011, 104, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Ford, K.R.; Harrington, C.A.; Clair, J.B. Photoperiod cues and patterns of genetic variation limit phenological responses to climate change in warm parts of species’ range: Modeling diameter-growth cessation in coast Douglas-fir. Glob. Chang. Biol. 2017, 23, 3348–3362. [Google Scholar] [CrossRef]
- Farook, U.B.; Khan, Z.H.; Ahad, I.; Aafreen, S.; Rafieq, I.; Yousuf, T.; Yousuf, W.; Jan, S.; Manzoor, A.; Wani, R. Screening for antixenosis resistance of winter wheat genotypes to cereal leaf beetles (Oulema melanopus L.). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3069–3073. [Google Scholar] [CrossRef]
- Zutter, N.D.; Audenaert, K.; Haesaert, G.; Smagghe, G. Preference of cereal aphids for different varieties of winter wheat. Arthropod Plant Interact. 2012, 6, 345–350. [Google Scholar] [CrossRef]
- Achhami, B.B.; Gadi, V.P.; Sherman, J.D.; Peterson, R.K.D.; Weaver, D.K. Antixenosis, antibiosis, and potential yield compensatory response in barley cultivars exposed to wheat stem sawfly (Hymenoptera: Cephidae) under field conditions. J. Insect Sci. 2020, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Piesik, D.; Rochat, D.; Pers, J.; Marion-Poll, F. Pulsed odors from maize or spinach elicit orientation in European corn borer neonate larvae. J. Chem. Ecol. 2009, 35, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Hondelmann, P.; Paul, C.; Schreiner, M.; Meyhofer, R. Importance of antixenosis and antibiosis resistance to the cabbage whitefly (Aleyrodes proletella) in brussels sprout cultivars. Insects 2020, 11, 56. [Google Scholar] [CrossRef] [Green Version]
- Girvin, J.; Whitworth, R.J.; Rojas, L.M.A.; Smith, C.M. Resistance of select winter wheat (Triticum aestivum) cultivars to Rhopalosiphum padi (Hemiptera: Aphididae). J. Econ. Entomol. 2017, 110, 1886–1889. [Google Scholar] [CrossRef]
- Anaii, M.M.; Yali, M.P. Resistance of nine wheat cultivars and lines to green bug, Schizaphis graminum (Rondani) in Iran. J. Agric. Sci. Technol. 2018, 20, 1173–1185. [Google Scholar]
- Wenda-Piesik, A.; Piesik, D.; Nowak, A.; Wawrzyniak, M. Tribolium confusum responses to blends of cereal kernels and plant volatiles. J. Appl. Entomol. 2016, 14, 558–563. [Google Scholar] [CrossRef]
- Piesik, D.; Wenda-Piesik, A. Sitophilus granarius responses to blends of five groups of cereal kernels and one group of plant volatiles. J. Stored Prod. Res. 2015, 62, 63–66. [Google Scholar] [CrossRef]
- Hu, X.; Liu, Y.; Wang, Y.; Wang, Z.; Yu, X.; Wang, B. Resistance of wheat accessions to the English grain aphid Sitobion avenae. PLoS ONE 2016, 11, e0156158. [Google Scholar] [CrossRef] [Green Version]
- Piesik, D.; Rochat, D.; Delaney, K.J.; Marionccoll, F. Orientation of European corn borer first instar larvae to synthetic green leaf volatiles. J. Appl. Entomol. 2013, 137, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Bruce, T.J.A.; Aradottir, G.I.; Smart, L.E.; Martin, J.L.; Caulfield, J.C.; Doherty, A.; Sparks, C.A.; Woodcock, C.M.; Birkett, M.A.; Napier, J.A. The first crop plant genetically engineered to release an insect pheromone for defense. Sci. Rep. 2015, 5, 11183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesler, L.S.; Riedell, W.E.; Kieckhefer, R.W.; Haley, S.D. Responses of Rhopalosiphum padi (Homoptera: Aphididae) on cereal aphid-resistant wheat accessions. J. Agric. Arban Entomol. 2002, 19, 133–140. [Google Scholar]
- Alkhedir, H.; Karlovsky, P.; Vidal, S. Relationship between water soluble carbohydrate content, aphid endosymbionts and clonal performance of Sitobion avenae on cocksfoot cultivars. PLoS ONE 2013, 8, e54327. [Google Scholar] [CrossRef] [Green Version]
- Staudt, M.; Jackson, B.; El-Aouni, H.; Buatois, B.; Lacroze, J.P.; Poessel, J.L.; Sauge, M.H. Volatile organic compound emissions induced by the aphid Myzus persicae differ among resistant and susceptible peach cultivars and a wild relative. Tree Physiol. 2010, 30, 1320–1334. [Google Scholar] [CrossRef] [Green Version]
- Hatano, E.; Kunert, G.; Bartram, S.; Boland, W.; Gershenzon, J.; Weisser, W.W. Do aphid colonies amplify their emission of alarm pheromone? J. Chem. Ecol. 2008, 34, 1149–1152. [Google Scholar] [CrossRef] [Green Version]
- Kunert, G.; Reinhold, C.; Gershenzon, J. Constitutive emission of the aphid alarm pheromone, (E)-b-farnesene, from plants does not serve as a direct defense against aphids. MBC Ecol. 2010, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Ninkovic, V.; Glinwood, R.; Unlu, A.G.; Ganji, S. Effects of methyl salicylate on host plant acceptance and feeding by the aphid Rhopalosiphum padi. Front. Plant Sci. 2021, 12, 1646. [Google Scholar] [CrossRef]
- Backus, E.A.; Cervantes, F.A.; Guedes, R.N.C.; Li, A.Y.; Wayadande, A.C. AC–DC electropenetrography for in-depth studies of feeding and oviposition behaviors. Ann. Entomol. Soc. Am. 2019, 112, 236–248. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Simon, A.; Halsey, K.; Kurup, S.; Clark, S.; Aradottir, G.I. Characterization of bird cherry-oat aphid (Rhopalosiphum padi L.) behavior and aphid host preference in relation to partially resistant and susceptible wheat landraces. Ann. Appl. Biol. 2020, 177, 184–194. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, H.; Hu, Z.; Li, D.; Zhang, Y. EPG comparison of Sitobion avenae (Fab.) feeding behavior on three wheat varieties. Agric. Sci. China 2008, 7, 180–186. [Google Scholar] [CrossRef]
- Greenslade, A.F.C.; Ward, J.L.; Martin, J.L.; Corol, D.I.; Clark, S.J.; Smart, L.E.; Aradottir, G.I. Triticum monococcum lines with distinct metabolic phenotypes and phloem-based partial resistance to the bird cherry-oat aphid Rhopalosiphum padi. Ann. Appl. Biol. 2016, 168, 435–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldin, E.L.L.; Stamm, M.D.; Bentivenha, J.P.F.; Koch, K.G.; Tiffany, M.; Hunt, T.E. Feeding behavior of Aphis glycines (Hemiptera: Aphididae) on soybeans exhibiting antibiosis, antixenosis, and tolerance resistance. BioOne 2018, 101, 223–228. [Google Scholar]
- Simon, A.L.; Caulfield, J.C.; Hammond-Kosack, K.E.; Field, L.M.; Aradottir, G.I. Identifying aphid resistance in the ancestral wheat Triticum monococcum under field conditions. Sci. Rep. 2021, 11, 13495. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, X.; Zhan, Y.; Wang, K.; Francis, F.; Liu, Y. New slow release mixture of (E)-β-farnesene with methyl salicylate to enhance aphid biocontrol efficacy in wheat ecosystem. Pest Manag. Sci. 2021, 77, 3341–3348. [Google Scholar] [CrossRef]
- Correa, L.D.J.; MacIel, O.V.B.; Bücker-Neto, L.; Pilati, L.; Morozini, A.M.; Faria, M.V.; Da-Silva, P.R. A Comprehensive analysis of wheat resistance to Rhopalosiphum padi (Hemiptera: Aphididae) in Brazilian wheat cultivars. J. Econ. Entomol. 2020, 113, 1493–1503. [Google Scholar] [CrossRef]
- Gyan, N.M.; Yaakov, B.; Weinblum, N.; Singh, A.; Cna’ani, A.; Ben-Zeev, S.; Saranga, Y.; Tzin, V. Variation between three Eragrostis tef accessions in defense responses to Rhopalosiphum padi aphid infestation. Front. Plant Sci. 2020, 70, 4011–4026. [Google Scholar] [CrossRef]
- Ni, X. Effect of wheat leaf epicuticular structure on host selection and probing rhythm of Russian wheat aphid (Homoptera: Aphididae). J. Econ. Entomol. 2014, 90, 1400–1407. [Google Scholar] [CrossRef]
- Leybourne, D.J.; Valentine, T.A.; Robertson, J.A.H.; Perez-fernandez, E.; Main, A.M.; Karley, A.J.; Bos, J.I.B. Defense gene expression and phloem quality contribute to mesophyll and phloem resistance to aphids in wild barley. J. Exp. Bot. 2019, 70, 4011–4026. [Google Scholar] [CrossRef]
- Singh, A.; Dilkes, B.; Sela, H.; Tzin, V. The effectiveness of physical and chemical defense responses of wild emmer wheat against aphids depends on leaf position and genotype. Front. Plant Sci. 2021, 12, 667820. [Google Scholar] [CrossRef]
- Khan, S.M. Varietal performance and chemical control used as tactics against sucking insect pests of cotton. Sarhad J. Agri. 2011, 27, 255–261. [Google Scholar]
- Li, F.; Kong, L.; Liu, Y.; Wang, H.; Chen, L.; Li, F.; Kong, L.; Liu, Y.; Wang, H.; Chen, L. Response of wheat germplasm to infestation of English grain aphid (Hemiptera: Aphididae) response of wheat germplasm to infestation of English grain aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2013, 106, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Board, E. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Painter, R. Insect Resistance in Crop Plants; Macmillan: New York, NY, USA, 1951; p. 638. [Google Scholar]
- Junaid, K.; Khan, S.A.; Khan, I.; Rizwan, S.; Shah, A.; Khan, Z. Study on different components of resistance in wheat genotypes to green gug (Schizaphis graminum) (Rondani). Pak. J. Zool. 2016, 48, 981–987. [Google Scholar]
- Tjallingii, W.F. A beginner’s guide to electronic monitoring of homopteran probing behavior. Principles and Applications of Electronic Monitoring and Other Techniques in the Study of Homopteran Feeding Behavior; Thomas Say Publications in Entomology, Entomological Society of America: Lanham, MD, USA, 2000; pp. 41–69. [Google Scholar]
- Sandanayaka, M.; Charles, J.G. Potential use of electrical penetration graph (EPG) technology for biosecurity incursion response decision making. N. Z. Plant Prot. 2017, 70, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; He, Y.; Lu, Z.; Chen, W.; Zhou, C.; Wang, X.; Li, T. An analysis of the feeding behavior of three stages of Toxoptera citricida by DC electrical penetration graph waveforms. Entomol. Exp. Appl. 2019, 167, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Sarria, E.; Cid, M.; Garzo, E.; Fereres, A. Excel workbook for automatic parameter calculation of EPG data. Comput. Electron. Agric. 2009, 67, 35–42. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive inalyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]



| No. | Wheat Accessions and Varieties | Resistance Scale | Aphid Index | Rank | |
|---|---|---|---|---|---|
| After 24 h | After 48 h | ||||
| 1 | 0 | 0 | 0 | 0.00 | Immunity |
| 2 | 3 | 1 | 1 | 0.01–0.30 | Highly resistance |
| 3 | 33 | 25 | 2 | 0.31–0.60 | Moderately resistance |
| 4 | 24 | 38 | 3 | 0.61–0.90 | Lowly resistance |
| 5 | 32 | 29 | 4 | 0.91–1.20 | Lowly susceptible |
| 6 | 27 | 19 | 5 | 1.21–1.50 | Moderately susceptible |
| 7 | 14 | 21 | 6 | >1.50 | Highly susceptible |
| Wheat Accessions and Varieties | Antixenosis Resistance | Antibiosis Resistance | ||
|---|---|---|---|---|
| Resistance Index | Resistance Description | Resistance Index | Resistance Description | |
| Lunxuan266 | 0.29 | HR | 0.67 | LR |
| 243726 | 0.36 | MR | 0.71 | LR |
| 213312 | 0.36 | MR | 1.13 | LS |
| 7276 | 0.43 | MR | 1.37 | MS |
| 243710 | 0.36 | MR | 0.67 | LR |
| 203971 | 0.43 | MR | 0.67 | LR |
| 7298 | 0.50 | MR | 1.46 | MS |
| 8324 | 0.36 | MR | 1.53 | HS |
| 7248 | 0.50 | MR | 1.08 | LS |
| 7407 | 0.50 | MR | 1.40 | MS |
| 243714 | 0.50 | MR | 0.64 | LS |
| 207845 | 0.43 | MR | 1.10 | LS |
| 227068 | 0.43 | MR | 0.73 | LR |
| Aphid Feeding Parameters | Wheat Accessions or Varieties Used for the EPG Recording | p-Value | |||
|---|---|---|---|---|---|
| 213312 (MR), n = 20 | Lunxuan266 (HR), n = 22 | 243726 (MR), n = 18 | Beijing 837 (HS), n = 14 | ||
| Number of Np | 3.55 ± 0.56 | 4.25 ± 0.44 | 4.62 ± 0.86 | 3.14 ± 0.76 | ns |
| Total duration of Np (min) | 76.99 ± 15.55 a | 119.06 ± 18.54 a | 75.48 ± 14.46 a | 54.8 ± 19.88 b | 0.0033 |
| Mean duration of Np (min) | 27.25 ± 7.01 | 31.47 ± 5.24 | 24.06 ± 6.74 | 30.32 ± 17.45 | ns |
| Total number of probes | 173.18 ± 20.45 | 148.10 ± 17.05 | 127.69 ± 22.56 | 150.50 ± 25.23 | ns |
| Time to the first probe (min) | 10.59 ± 3.75 ab | 25.18 ± 7.24 a | 23.77 ± 7.32 a | 6.85 ± 2.24 b | 0.0449 |
| Total probing time (h) | 4.59 ± 0.25 | 4.13 ± 0.32 | 4.74 ± 0.24 | 5.04 ± 0.32 | ns |
| Duration of first probe (min) | 2.62 ± 0.49 ab | 7.84 ± 4.02 a | 3.35 ± 1.21 ab | 1.58 ± 0.35 b | 0.0440 |
| Number of C | 85.64 ± 1.77 | 74.55 ± 1.81 | 63.92 ± 1.70 | 72.79 ± 1.79 | ns |
| Total duration of C (h) | 2.01 ± 0.21 | 1.91 ± 0.18 | 1.91 ± 0.19 | 1.47 ± 0.22 | ns |
| Mean duration of C (min) | 1.50 ± 0.19 | 2.38 ± 0.59 | 1.99 ± 0.42 | 1.40 ± 0.20 | ns |
| Number of Pd | 77.93 ± 9.52 | 67.65 ± 7.95 | 54.69 ± 9.81 | 67.93 ± 11.97 | ns |
| Total duration of Pd (min) | 7.39 ± 0.87 | 13.04 ± 5.52 | 4.71 ± 0.87 | 7.08 ± 1.38 | ns |
| Number of G | 2.27 ± 0.40 | 1.45 ± 0.21 | 2.00 ± 0.27 | 2.21 ± 0.59 | ns |
| Total number of G (min) | 57.79 ± 12.21 | 50.54 ± 11.02 | 99.01 ± 18.41 | 37.00 ± 8.95 | ns |
| Mean number of G (min) | 23.46 ± 4.25 | 34.04 ± 9.13 | 57.85 ± 15.90 | 20.16 ± 6.58 | ns |
| Time to the first E1 (h) | 2.23 ± 0.34 | 2.91 ± 0.43 | 3.05 ± 0.60 | 1.73 ± 0.25 | ns |
| Number of E1 | 5.14 ± 0.73 | 3.50 ± 0.61 | 5.08 ± 1.48 | 4.14 ± 0.71 | ns |
| Total duration of E1(min) | 46.16 ± 7.01 | 43.01 ± 7.12 | 38.51 ± 8.13 | 29.79 ± 9.49 | ns |
| Mean duration of E1 (min) | 13.89 ± 3.41 a | 9.46 ± 1.91 a | 8.98 ± 1.79 ab | 3.87 ± 0.83 b | 0.0360 |
| Time to the first E2 | 3.35 ± 0.43 ab | 4.64 ± 0.45 a | 3.61 ± 0.59 ab | 2.36 ± 0.34 b | 0.0409 |
| Number of E2 | 2.22 ± 0.49 ab | 0.95 ± 0.31 b | 2.00 ± 0.65 ab | 3.43 ± 0.73 a | 0.0063 |
| Total duration of E2 (min) | 49.92 ± 12.55 b | 31.22 ± 13.35 b | 59.68 ± 20.37 b | 126.99 a | 0.0020 |
| Mean duration of E2 (min) | 24.55 ± 8.84 b | 11.80 ± 5.09 b | 25.39 ± 10.70 b | 52.48 ± 17.20 a | 0.0061 |
| Time to first sustained E2 (h) | 4.27 ± 0.41 ab | 5.21 ± 0.28 a | 4.81 ± 0.60 ab | 3.45 ± 0.42 b | 0.0474 |
| EPG Parameters | Percentage of Winged Aphids Settled on Wheat Accessions or Varieties after Different Infestation Times | |||
|---|---|---|---|---|
| 12 h | 24 h | 48 h | 72 h | |
| Total duration of Np (min) | −0.31 * | −0.62 *** | −0.29 * | −0.34 * |
| Total duration of C (h) | −0.13 | −0.17 | −0.17 | −0.17 |
| Total duration of Pd (min) | −0.06 | −0.08 | −0.07 | −0.09 |
| Mean duration of E1(min) | −0.27 * | −0.31 * | −0.28 * | −0.27 * |
| Total duration of E2 (min) | 0.40 * | 0.53 *** | 0.48 *** | 0.50 *** |
| Total number of G (min) | 0.14 | 0.12 | 0.17 | 0.07 |
| Total duration of Np (min) | −0.31 * | −0.62 *** | −0.29 * | −0.34 * |
| Trichome Density (mm2) | Percentage of Winged Aphids Settled on Wheat Accessions after Different Infestation Times | |||
|---|---|---|---|---|
| 12 h | 24 h | 48 h | 72 h | |
| Adaxial trichomes | −0.53 *** | −0.58 *** | −0.56 *** | −0.58 *** |
| Abaxial trichomes | −0.44 ** | −045 ** | −0.48 ** | −0.47 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebretsadik, K.G.; Zhang, Y.; Chen, J. Screening and Evaluation for Antixenosis Resistance in Wheat Accessions and Varieties to Grain Aphid, Sitobion miscanthi (Takahashi) (Hemiptera: Aphididae). Plants 2022, 11, 1094. https://doi.org/10.3390/plants11081094
Gebretsadik KG, Zhang Y, Chen J. Screening and Evaluation for Antixenosis Resistance in Wheat Accessions and Varieties to Grain Aphid, Sitobion miscanthi (Takahashi) (Hemiptera: Aphididae). Plants. 2022; 11(8):1094. https://doi.org/10.3390/plants11081094
Chicago/Turabian StyleGebretsadik, Kifle Gebreegziabiher, Yong Zhang, and Julian Chen. 2022. "Screening and Evaluation for Antixenosis Resistance in Wheat Accessions and Varieties to Grain Aphid, Sitobion miscanthi (Takahashi) (Hemiptera: Aphididae)" Plants 11, no. 8: 1094. https://doi.org/10.3390/plants11081094