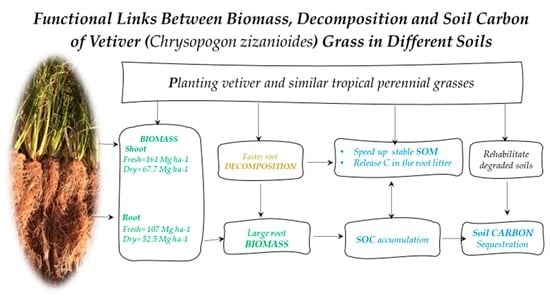
Functional Links between Biomass Production and Decomposition of Vetiver (Chrysopogon zizanioides) Grass in Three Australian Soils
Abstract
:1. Introduction
2. Results
2.1. Vetiver Biomass Production
2.2. Vetiver Root and Shoot Relative Decomposition Rate
2.3. Effect of Soil Type on Carbon Evolved from the Vetiver Biomass
2.3.1. Carbon Evolved from Three Soil Types
2.3.2. Effect of Soil Type on Carbon Evolved from Vetiver Shoots
2.3.3. Effect of Soil Type on Total Carbon Evolved from Vetiver Roots
3. Discussion
3.1. Vetiver Biomass Production
3.2. Vetiver Biomass Decomposition
4. Materials and Methods
4.1. Experimental Setting and Design
4.2. Treatment Description
4.3. Sample Collection, Preparation & Analysis
4.3.1. Biomass Production
4.3.2. Decomposition
4.4. Data Analysis
5. Conclusions and Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Terefe, H.; Argaw, M.; Tamene, L.; Mekonnen, K. Sustainable land management interventions lead to carbon sequestration in plant biomass and soil in a mixed crop-livestock system: The case of Geda watershed, central highlands of Ethiopia. Ecol. Process. 2020, 9, 34. [Google Scholar] [CrossRef]
- Liang, X.; Erickson, J.E.; Silveira, M.L.; Sollenberger, L.E.; Rowland, D.L.; Vermerris, W. Quantifying shoot and root biomass production and soil carbon under perennial bioenergy grasses in a subtropical environment. Biomass-Bioenergy 2019, 128, 105323. [Google Scholar] [CrossRef]
- Reichle, D.; Houghton, J.; Kane, B.; Ekmann, J.; Benson, S.; Clarke, J.; Dahlman, R.; Hendrey, G.; Herzog, H.; Huntercevera, J. Carbon Sequestration: State of the Science; US Department of Energy, Offices of Science and Fossil Energy: Washington, DC, USA, 1999.
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- McKenzie, D.; Mason, W. Soil Carbon Sequestration under Pasture in Southern Australia. Inquiry into Soil Sequestration in Victoria; Submission no. 29 Prepared for Dairy Australia, Project MCK13538; Dairy Australia: Southbank, Australia, 2010. [Google Scholar]
- Fisher, M.J.; Braz, S.; Dos Santos, R.; Urquiaga, S.; Alves, B.; Boddey, R. Another dimension to grazing systems: Soil carbon. Trop. Grassl. 2007, 41, 65. [Google Scholar]
- Fisher, M.J.; Rao, I.M.; Ayarza, M.A.; Lascano, C.E.; Sanz, J.I.; Thomas, R.J.; Vera, R.R. Carbon storage by introduced deep-rooted grasses in the South American savannas. Nature 1994, 371, 236–238. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Review: Factors affecting rhizosphere priming effects. J. Plant Nutr. Soil Sci. 2002, 165, 382–396. [Google Scholar] [CrossRef]
- Nguyen, C. Rhizodeposition of organic C by plants: Mechanisms and controls. Agronomie 2003, 23, 375–396. [Google Scholar] [CrossRef]
- Kell, D.B. Breeding crop plants with deep roots: Their role in sustainable carbon, nutrient and water sequestration. Ann. Bot. 2011, 108, 407–418. [Google Scholar] [CrossRef] [Green Version]
- Monti, A.; Zatta, A. Root distribution and soil moisture retrieval in perennial and annual energy crops in Northern Italy. Agric. Ecosyst. Environ. 2009, 132, 252–259. [Google Scholar] [CrossRef]
- Kelbessa, E.; Mengistou, S.; Getahun, A. Wetland and Aquatic Resource of Ethiopia: Status, Challenges and Prospects; Biological Society of Ethiopia: Addis Ababa, Ethiopia, 2004. [Google Scholar]
- Zatta, A.; Clifton-Brown, J.; Robson, P.; Hastings, A.; Monti, A. Land use change from C3 grassland to C4 Miscanthus: Effects on soil carbon content and estimated mitigation benefit after six years. Global Chang. Biol. Bioenergy 2013, 6, 360–370. [Google Scholar] [CrossRef] [Green Version]
- Bills, J.S.; Jacinthe, P.-A.; Tedesco, L.P. Soil organic carbon pools and composition in a wetland complex invaded by reed canary grass. Biol. Fertil. Soils 2010, 46, 697–706. [Google Scholar] [CrossRef]
- Scherer-Lorenzen, M.; Bonilla, J.L.; Potvin, C. Tree species richness affects litter production and decomposition rates in a tropical biodiversity experiment. Oikos 2007, 116, 2108–2124. [Google Scholar] [CrossRef]
- Walela, C.; Daniel, H.; Wilson, B.; Lockwood, P.; Cowie, A.; Harden, S. The initial lignin:nitrogen ratio of litter from above and below ground sources strongly and negatively influenced decay rates of slowly decomposing litter carbon pools. Soil Biol. Biochem. 2014, 77, 268–275. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Van Veen, J.A.; Kuikman, P.J. Soil structural aspects of decomposition of organic matter by micro-organisms. Biodegradation 1990, 11, 213–233. [Google Scholar] [CrossRef]
- Van Groenigen, K.J.; Qi, X.; Osenberg, C.W.; Luo, Y.; Hungate, B.A. Faster Decomposition Under Increased Atmospheric CO2 Limits Soil Carbon Storage. Science 2014, 344, 508–509. [Google Scholar] [CrossRef]
- De Wit, M.P.; Lesschen, J.P.; Londo, M.H.; Faaij, A.P. Greenhouse gas mitigation effects of integrating biomass production into European agriculture. Biofuels, Bioprod. Biorefining 2014, 8, 374–390. [Google Scholar] [CrossRef]
- Chendev, Y.G.; Novykh, L.L.; Sauer, T.J.; Petin, A.N.; Zazdravnykh, E.A.; Burras, C.L. Evolution of Soil Carbon Storage and Morphometric Properties of Afforested Soils in the US Great Plains Soil Carbon; Springer: Cham, Switzerland, 2014; pp. 475–482. [Google Scholar]
- Scheffer, R.A.; Aerts, R. Root decomposition and soil nutrient and carbon cycling in two temperate fen ecosystems. Oikos 2000, 91, 541–549. [Google Scholar] [CrossRef]
- Amougou, N.; Bertrand, I.; Cadoux, S.; Recous, S. Miscanthus × giganteusleaf senescence, decomposition and C and N inputs to soil. GCB Bioenergy 2012, 4, 698–707. [Google Scholar] [CrossRef]
- Beuch, S.; Boelcke, B.; Belau, L. Effect of the Organic Residues of Miscanthus x giganteus on the Soil Organic Matter Level of Arable Soils. J. Agron. Crop Sci. 2000, 184, 111–120. [Google Scholar] [CrossRef]
- Singh, G.; Dagar, J. Biosaline agriculture: Perspective and opportunities. J. Soil Salin. Water Qual. 2009, 1, 41–49. [Google Scholar]
- Singh, M.; Guleria, N.; Rao, E.V.S.P.; Goswami, P. Efficient C sequestration and benefits of medicinal vetiver cropping in tropical regions. Agron. Sustain. Dev. 2014, 34, 603–607. [Google Scholar] [CrossRef] [Green Version]
- Gaspard, S.; Altenor, S.; Dawson, E.; Barnes, P.; Ouensanga, A. Activated carbon from vetiver roots: Gas and liquid adsorption studies. J. Hazard. Mater. 2007, 144, 73–81. [Google Scholar] [CrossRef]
- Lavania, S. Vetiver root system: Search for the ideotype. In Proceedings of the Third International Conference on Vetiver and Exhibition, Guangzhou, China, 6–9 October 2003. [Google Scholar]
- Tomar, O.; Minhas, P. Relative performance of aromatic grasses under saline irrigation. Indian J. Agron. 2004, 49, 207–208. [Google Scholar]
- Neal, J.S.; Fulkerson, W.J.; Lawrie, R.; Barchia, I.M. Difference in yield and persistence among perennial forages used by the dairy industry under optimum and deficit irrigation. Crop Pasture Sci. 2009, 60, 1071–1087. [Google Scholar] [CrossRef]
- Kaveeta, L.; Sopa, R.; Na, M.; Nakorn, R.K.; Nakorn, W.N.; Charoenrungrat, W. Developmental Variation of Four Selected Vetiver Ecotypes. In Proceedings of the Second International Conference on Vetiver: Vetiver and the Environment, Bangkok, Thailand, 1–20 January 2002. [Google Scholar]
- Xu, L. Simple treatment to protect vetiver against cold winter in north subtropics of China. Vetiverim 2005, 33, 21–23. [Google Scholar]
- Chairoj, P.; Roongtanakiat, N. Decomposition of Vetiver Shoot and Effect of Vetiver Mulching on Super Sweet Corn Hybrid Yield. Kasetsart J. (Nat. Sci.) 2004, 38, 305–310. [Google Scholar]
- Percy, I.; Truong, P. Landfill leachate disposal with irrigated vetiver grass. In Proceedings of the Third International Conference on Vetiver and Exhibition (ICV-3), Guangzhou, China, 6–9 October 2003. [Google Scholar]
- Wagner, S.; Truong, P.; Vieritz ASmeal, C. Response of vetiver grass to extreme nitrogen and phosphorus supply. In Proceedings of the Third International Conference on Vetiver and Exhibition, Guangzhou, China, 6–9 October 2003. [Google Scholar]
- Liu, J.; Jiang, P.; Wang, H.; Zhou, G.; Wu, J.; Yang, F.; Qian, X. Seasonal soil CO2 efflux dynamics after land use change from a natural forest to Moso bamboo plantations in subtropical China. For. Ecol. Manag. 2011, 262, 1131–1137. [Google Scholar] [CrossRef]
- Gijsman, A.; Alarcón, H.; Thomas, R. Root decomposition in tropical grasses and legumes, as affected by soil texture and season. Soil Biol. Biochem. 1997, 29, 1443–1450. [Google Scholar] [CrossRef]
- Sanaullah, M.; Chabbi, A.; Leifeld, J.; Bardoux, G.; Billou, D.; Rumpel, C. Decomposition and stabilization of root litter in top- and subsoil horizons: What is the difference? Plant Soil 2011, 338, 127–141. [Google Scholar] [CrossRef]
- Dalal, R.C.; Thornton, C.; Cowie, B.A. Turnover of organic carbon and nitrogen in soil assessed from δ13C and δ15N changes under pasture and cropping practices and estimates of greenhouse gas emissions. Sci. Total Environ. 2013, 465, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novara, A.; Gristina, L.; Kuzyakov, Y.; Schillaci, C.; Laudicina, V.A.; La Mantia, T. Turnover and availability of soil organic carbon under different Mediterranean land-uses as estimated by13C natural abundance. Eur. J. Soil Sci. 2013, 64, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Solly, E.F.; Schöning, I.; Boch, S.; Kandeler, E.; Marhan, S.; Michalzik, B.; Müller, J.; Zscheischler, J.; Trumbore, S.E.; Schrumpf, M. Factors controlling decomposition rates of fine root litter in temperate forests and grasslands. Plant Soil 2014, 382, 203–218. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Shi, Z.; Jiang, J.; Xia, J.; Liang, J.; Zhou, J.; Luo, Y. Methodological uncertainty in estimating carbon turnover times of soil fractions. Soil Biol. Biochem. 2016, 100, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Poeplau, C.; Don, A.; Six, J.; Kaiser, M.; Benbi, D.; Chenu, C.; Cotrufo, M.F.; Derrien, D.; Gioacchini, P.; Grand, S.; et al. Isolating organic carbon fractions with varying turnover rates in temperate agricultural soils—A comprehensive method comparison. Soil Biol. Biochem. 2018, 125, 10–26. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Halliday, M.; Gifford, R. Fine root decomposition under grass and pine seedlings in controlled environmental conditions. Appl. Soil Ecol. 2006, 33, 22–29. [Google Scholar] [CrossRef]
- Li, X.; Hu, F.; Shi, W. Plant material addition affects soil nitrous oxide production differently between aerobic and oxygen-limited conditions. Appl. Soil Ecol. 2013, 64, 91–98. [Google Scholar] [CrossRef]
- Vinten, A.J.; Whitmore, A.; Bloem, J.; Howard, R.; Wright, F. Factors affecting N immobilisation/mineralisation kinetics for cellulose-, glucose-and straw-amended sandy soils. Biol. Fertil. Soils 2002, 36, 190–199. [Google Scholar] [CrossRef]
- Rousk, J.E. Fungal and bacterial growth in soil with plant materials of different C/N ratios. FEMS Microbiol. Ecol. 2007, 62, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Lakshmanaperumalsamy, P.; Jayashree, S.; Rathinamala, J. Biomass Production of Vetiver (Vetiveria zizanioides) Using Vermicompost; Bharathiar University: Coimbatore, India, 2006. [Google Scholar]
- Lavania, U.; Lavania, S. Sequestration of atmospheric carbon into subsoil horizons through deep-rooted grasses-vetiver grass model. Curr. Sci. 2009, 97, 618–619. [Google Scholar]
- Wattanaprapat, K.; Meesing, K.K.I.; Nopmalai, P. Correlation between Vetiver Root Biomass with Soil Organic Carbon and CO2 Emission in Agricultural Areas of the Southern Part of Thailand; Land Development Department: Bangkok, Thailand, 2006. [Google Scholar]
- Wang, X.; Butterly, C.R.; Baldock, J.A.; Tang, C. Long-term stabilization of crop residues and soil organic carbon affected by residue quality and initial soil pH. Sci. Total Environ. 2017, 587–588, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, F.; Saliendra, N.; Crisosto, C. Carbon Isotope Discrimination and Gas Exchange in Coffea arabica During Adjustment to Different Soil Moisture Regimes. Funct. Plant Biol. 1992, 19, 171–184. [Google Scholar] [CrossRef]
- Isbell, R. The Australian Soil Classification; CSIRO Publishing: Canberra, Australia, 2016. [Google Scholar]
- Isbell, R.F. The Australian Soil Classification; CSIRO Publishing: Melbourne, Australia, 2002. [Google Scholar]
- Milne, E.; Banwart, S.A.; Noellemeyer, E.; Abson, D.J.; Ballabio, C.; Bampa, F.; Bationo, A.; Batjes, N.H.; Bernoux, M.; Bhattacharyya, T.; et al. Soil carbon, multiple benefits. Environ. Dev. 2015, 13, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Krull, E.S.; Bestland, E.A.; Gates, W.P. Soil Organic Matter Decomposition and Turnover in a Tropical Ultisol: Evidence from δ13C, δ15N and Geochemistry. Radiocarbon 2002, 44, 93–112. [Google Scholar] [CrossRef] [Green Version]
- Don, A.; Kalbitz, K. Amounts and degradability of dissolved organic carbon from foliar litter at different decomposition stages. Soil Biol. Biochem. 2005, 37, 2171–2179. [Google Scholar] [CrossRef]
- Garnett, T.; Godde, C.; Muller, A.; Röös, E.; Smith, P.; Boer, I.D.; Ermgassen, E.Z.; Herrero, M.; Middelaar, C.V.; Schader, C.; et al. Grazed and Confused? Ruminating on Cattle, Grazing Systems, Methane, Nitrous Oxide, the Soil Carbon Sequestration Question—And What It All Means for Greenhouse Gas Emissions; Oxford Martin School: Oxford, UK, 2017. [Google Scholar]





| Plant Allocation | Fresh Biomass (kg m−3) | Dry Biomass (kg m−3) |
|---|---|---|
| Above-ground biomass | 1.61 ± 0.218 | 0.67 ± 0.101 |
| Below-ground biomass | 1.07 ± 0.128 | 0.53 ± 0.054 |
| Total biomass | 2.68 ± 0.344 | 1.2 ± 0.151 |
| Shoot−to−Root ratio | 1:1.43 | 1:1.25 |
| Root Depth (cm) | Fresh (g) | Dry (g) |
|---|---|---|
| 0–10 | 56.2 ± 29.9 | 28.9 ± 13.5 |
| 10–20 | 17.7 ± 7.1 | 9.4 ± 1.7 |
| 20–30 | 14.6 ± 6.6 | 7.1 ± 2.8 |
| 30–40 | 12.5 ± 6.0 | 5.3 ± 2.7 |
| 40–50 | 8.4 ± 4 | 3.6 ± 1.8 |
| 50–70 | 9.3 ± 7.8 | 3.7 ± 3.2 |
| >70 | 2.8 ± 5.4 | 1.6 ± 3.1 |
| a | b | R2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | Sand | Silt | Clay | Sand | Silt | Clay | |
| Soil | 210 ± 45 | 256 ± 43 | 400 ± 109 | −0.04 ± 0.015 | −0.02 ± 0.006 | −0.04 ± 0.02 | 0.53 | 0.53 | 0.49 |
| Shoot | 186 ± 43 | 342 ± 44 | 484 ± 159 | −0.03 ± 0.013 | −0.02 ± 0.005 | −0.06 ± 0.031 | 0.47 | 0.68 | 0.51 |
| Root | 177 ± 23 | 224 ± 17 | 830 ± 104 | −0.05 ± 0.01 | –0.02 ± 0.003 | −0.1 ± 0.014 | 0.35 | 0.42 | 0.64 |
| Root Depth (cm) | a | b | R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | Sand | Silt | Clay | Sand | Silt | Clay | |
| 0–10 | 364 ± 125 | 225 ± 48.5 | 756 ± 173 | −0.090 ± 0.04 | −0.021 ± 0.01 | −0.074 ± 0.02 | 0.55 | 0.44 | 0.74 |
| 10–20 | 355 ± 110 | 198 ± 50.5 | 967 ± 466 | −0.090 ± 0.03 | −0.020 ± 0.01 | −0.141 ± 0.06 | 0.62 | 0.35 | 0.6 |
| 20–30 | 58 ± 12.7 | 226 ± 38.8 | 919 ± 301 | −0.020 ± 0.01 | −0.020 ± 0.01 | −0.097 ± 0.04 | 0.42 | 0.54 | 0.64 |
| 30–40 | 399 ± 212 | 324 ± 53.2 | 1130 ± 595 | −0.130 ± 0.06 | −0.031 ± 0.01 | −0.172 ± 0.07 | 0.42 | 0.65 | 0.64 |
| 40–50 | 142.0 ± 3 | 305 ± 46.7 | 791 ± 230 | −0.021 ± 0.01 | −0.015 ± 0.01 | −0.095 ± 0.03 | 0.41 | 0.54 | 0.7 |
| 50–70 | 242 ± 49 | 184 ± 44.3 | 734 ± 232 | −0.032 ± 0.01 | −0.015 ± 0.01 | −0.085 ± 0.03 | 0.55 | 0.31 | 0.62 |
| 70–100 | 115 ± 2.6 | 144 ± 33.0 | 764 ± 234 | −0.033 ± 0.01 | −0.013 ± 0.01 | −0.086 ± 0.03 | 0.65 | 0.32 | 0.63 |
| 0–100 | 177 ± 23 | 224 ± 17.0 | 830 ± 104 | −0.500 ± 0.01 | −0.02 ± 0.003 | −0.1 ± 0.014 | 0.35 | 0.42 | 0.64 |
| Soil Type | Texture | Sand % | Silt % | Clay % | SOC % | δ13C % | % TN | δ15N | C:N | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| Sandy-Chromosol | Sandy loam | 76.30 | 10.50 | 13.20 | 1.06 | −18.39 | 0.09 | 0.37 | 0.09 | 4.4 |
| Silty-Chromosol | Silty clay loam | 45.00 | 25.00 | 30.00 | 2.25 | −19.80 | 0.20 | 0.37 | 0.44 | 5.2 |
| Clayey-Dermosol | Silty clay | 19.00 | 26.00 | 55.00 | 2.03 | −19.23 | 0.14 | 0.37 | 0.29 | 6.4 |
| Experiment | No. of Pots | Reps | Biomass | Materials Used | Activity | Analysis |
|---|---|---|---|---|---|---|
| Biomass production | 5 | 5 | Fresh | Shoot and Root | Harvesting | Measure and weighing |
| 5 1 | Dry | Shoot and Root | ||||
| Decomposition 2 | 2 | 4 | Fresh | Shoot | Incubation | GC-MS |
| 4 × 7 depth | Fresh | Roots | ||||
| 4 | None | Soils |
| Vetiver | C (%) | δ13C | N (%) | C:N |
|---|---|---|---|---|
| Shoot | 44.10 | −11.59 | 1.49 | 66 |
| Root | 34.22 | −13.87 | 0.61 | 21 |
| Sand | Silt | Clay | Soil Type | Rep | Depth | Total Sample | |
|---|---|---|---|---|---|---|---|
| Blank | - | - | - | - | 4 | - | 4 |
| Soil | Sand | Silt | Clay | 3 | 4 | - | 12 |
| Shoot | Shoot + Sand | Shoot + Silt | Shoot + Clay | 3 | 4 | - | 12 |
| Root | (Root * + Sand) | Root * + Silt | Root * + Clay | 3 | 4 | 7 | 84 |
| root (0–10 cm) + sand | root (0–10 cm) + silt | root (0–10 cm) + clay | |||||
| root (10–20 cm) + sand | root (10–20 cm) + silt | root (10–20 cm) + clay | |||||
| root (20–30 cm) + sand | root (20–30 cm) + silt | root (20–30 cm) + clay | |||||
| root (30–40 cm) + sand | root (30–40 cm) + silt | root (30–40 cm) + clay | |||||
| root (40–50 cm) + sand | root (40–50 cm) + silt | root (40–50 cm) + clay | |||||
| root (50–70 cm) + sand | root (50–70 cm) + silt | root (50–70 cm) + clay | |||||
| root (>70 cm) + sand | root (>70 cm) + silt | root (>70 cm) + clay | |||||
| Total no. of samples | 112 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessema, B.; Wilson, B.; Daniel, H.; Kristiansen, P.; Baldock, J.A. Functional Links between Biomass Production and Decomposition of Vetiver (Chrysopogon zizanioides) Grass in Three Australian Soils. Plants 2022, 11, 778. https://doi.org/10.3390/plants11060778
Tessema B, Wilson B, Daniel H, Kristiansen P, Baldock JA. Functional Links between Biomass Production and Decomposition of Vetiver (Chrysopogon zizanioides) Grass in Three Australian Soils. Plants. 2022; 11(6):778. https://doi.org/10.3390/plants11060778
Chicago/Turabian StyleTessema, Bezaye, Brian Wilson, Heiko Daniel, Paul Kristiansen, and Jeff A. Baldock. 2022. "Functional Links between Biomass Production and Decomposition of Vetiver (Chrysopogon zizanioides) Grass in Three Australian Soils" Plants 11, no. 6: 778. https://doi.org/10.3390/plants11060778