Waterlogged Conditions Influence the Nitrogen, Phosphorus, Potassium, and Sugar Distribution in Sago Palm (Metroxylon sagu Rottb.) at Seedling Stages
Abstract
:1. Introduction
2. Results
2.1. Morphological Growth Traits and Root Morphology for Adaptation
2.2. Sugar Concentration in Petioles and Roots
2.3. Nutrient Concentration in Each Plant Organ
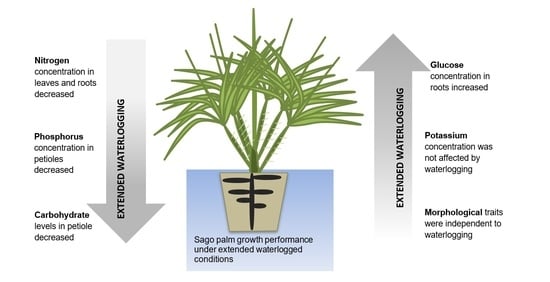
3. Discussion
4. Materials and Methods
4.1. Experimental Location and Materials
4.2. Morphological Growth Traits
4.3. Carbohydrate Analysis
4.4. Nitrogen, Phosphorus, and Potassium Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crawford, R.M.M.; Braendle, R. Oxygen deprivation stress in a changing environment. J. Exp. Bot. 1996, 47, 145–159. [Google Scholar] [CrossRef]
- Bansal, R.; Srivastava, J.P. Effect of waterlogging on photosynthetic and biochemical parameters in pigeonpea. Russ. J. Plant Physiol. 2015, 62, 322–327. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ando, H.; Watanabe, A.; Kakuda, K.; Jong, F.S.; Jamallam, L. Effects of Groundwater Level and Fertilizer Application in Sustainable Sago Palm Cultivation in Tropical Peat; JSPS Report; Yamagata University: Tsuruoka, Japan, 2007. [Google Scholar]
- Azhar, A.; Makihara, D.; Naito, H.; Ehara, H. Evaluating sago palm (Metroxylon sagu Rottb.) photosynthetic performance in waterlogged conditions: Utilizing pulse-amplitude-modulated (PAM) fluorometry as a waterlogging stress indicator. J. Saudi Soc. Agric. Sci. 2018, 19, 37–42. [Google Scholar] [CrossRef]
- Ehara, H. Geographycal distributon and specification of Metroxylon palms. Jpn. J. Trop. Agric. 2005, 50, 229–233. [Google Scholar]
- Asano, K.; Isoi, T.; Murano, H.; Azhar, A.; Pasolon, Y.B.; Ehara, H. Colonization of Roots in Sago Palm Seedlings Associated with Commercial Mycorrhizal inocula. Sago Palm. 2019, 27, 9–14. [Google Scholar]
- Mendez, Y.D.R.; Cuenca, J.C.; Romero, H.M. Physiological responses of oil palm (Elaeis guineensis Jacq.) seedlings under different water soil conditions. Agron. Colomb. 2016, 34, 163–171. [Google Scholar]
- Misra, V.; Solomon, S.; Singh, P.; Prajapati, C.P.; Ansari, M.I. Effect of Water Logging on Post-harvest Sugarcane Deterioration. Agrica 2016, 5, 119–132. [Google Scholar] [CrossRef]
- Purwanto, B.H.; Kakuda, K.I.; Ando, H.; Shoon, J.F.; Yamamoto, Y.; Watanabe, A.; Yoshida, T. Nutrient availability and response of sago palm (Metroxylon sagu Rottb.) to controlled release n fertilizer on coastal lowland peat in the tropics. Soil Sci. Plant Nutr. 2002, 48, 529–537. [Google Scholar] [CrossRef]
- Najeeb, U.; Bange, M.P.; Tan, D.K.Y.; Atwell, B.J. Consequences of waterlogging in cotton and opportunities for mitigation of yield losses. AoB Plants 2014, 7, plv080. [Google Scholar] [CrossRef]
- Yamamoto, Y. Sago Palm: Tropical Agricultural Series; Assosiation for International Cooperation Agriculture and Forestry: Tokyo, Japan, 1998. [Google Scholar]
- Nitta, Y.; Goto, Y.; Kakuda, K.; Ehara, H.; Ando, H.; Yoshida, T.; Yamamoto, Y.; Matsuda, T.; Jong, F.S.; Hasan, A.H. Morphological observants of adventitious and lateral roots of sago palm. Plant Prod. Sci. 2002, 5, 139–145. [Google Scholar] [CrossRef]
- Azhar, A.; Audia, F.; Asano, K.; Makihara, D.; Naito, H.; Sugiura, D.; Ehara, H. The impact of different soil water levels on nitrogen, phosphorus, and potassium uptake, photosynthetic performance, and sugar distribution of sago palm seedlings. Agrosystems Geosci. Environ. 2021, 4, e20191. [Google Scholar] [CrossRef]
- Aluko, O.O.; Li, C.; Wang, Q.; Liu, H. Sucrose Utilization for Improved Crop Yields: A Review Article. Int. J. Mol. Sci. 2021, 22, 4704. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Li, F.; Chen, X.; Xie, Y. Growth responses and non-structural carbohydrates in three wetland macrophyte species following submergence and de-submergence. Acta Physiol. Plant. 2013, 35, 2069–2074. [Google Scholar] [CrossRef]
- Borella, J.; Amarante, L.D.; Oliveira, D.D.S.C.D.; Oliveira, A.C.B.D.; Braga, E.J.B. Waterlogging-induced changes in fermentative metabolism in roots and nodules of soybean genotypes. Sci. Agric. 2014, 71, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Zanandrea, I.; Alves, J.D.; Deuner, S.; Goulart, P.F.P.G.; Henrique, P.C.; Silveria, N.M. Tolerance of Sesbania virgata plants to flooding. Aust. J. Bot. 2010, 57, 661–669. [Google Scholar] [CrossRef]
- Silveira, H.R.O.; Souza, K.R.D.; Alves, J.D.; Santos, M.O.; Andrade, C.A.; Bomfim, S.C. Gas exchange and carbohydrate partitioning in coffee seedlings under waterlogging. Ciênc. Agrotec. 2015, 39, 138–146. [Google Scholar] [CrossRef]
- Jackson, M.B.; Saker, L.R.; Crisp, C.M.; Else, M.A.; Janowiak, F. Ionic and pH signalling from roots to shoots of flooded tomato plants in relation to stomatal closure. Plant Soil. 2003, 253, 103–113. [Google Scholar] [CrossRef]
- Steffens, D.; Hutsch, B.; Eschholz, T.; Losak, T.; Schubert, S. Water logging may inhibit plant growth primarily by nutrient deficiency rather than nutrient toxicity. Plant Soil Environ. 2005, 51, 545. [Google Scholar] [CrossRef] [Green Version]
- Colmer, T.D.; Greenway, H. Ion transport in seminal and adventitious roots of cereals during O2 deficiency. J. Exp. Bot. 2011, 62, 39–57. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.; Cao, X.; Hu, J.; Zhu, L.; Zhang, J.; Huang, J.; Jin, Q. Nitrogen metabolism in adaptation of photosynthesis to water stress in rice grown under different nitrogen levels. Front. Plant Sci. 2017, 8, 1079. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.W.; Qiang, L.; Rong, J.; Wei, C.; Liu, X.L.; Kong, F.; Ke, Y.; Shi, H.; Yuan, J. Effect of low-nitrogen stress on photosynthesis and chlorophyll fluorescence characteristics of maize cultivars with different lownitrogen tolerances. J. Integr. Agric. 2019, 18, 1246–1256. [Google Scholar] [CrossRef]
- Azhar, A.; Makihara, D.; Naito, H.; Ehara, H. Photosynthesis of sago palm (Metroxylon sagu Rottb.) seedling at different air temperatures. Agriculture 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Rennenberg, H.; Kreuzwieser, J. Hypoxia affects nitrogen uptake and distribution in young poplar (Populus × canescens) trees. PLoS ONE 2015, 10, e136579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey-Serres, J.; Voesenek, L. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, I.M.; Terry, N. Leaf phosphate status, photosynthesis and carbon partitioning in sugar beet. 4. changes with time following increased supply of phosphate to low-phosphate plants. Plant Physiol. 1995, 107, 1313–1321. [Google Scholar] [CrossRef] [Green Version]
- Ehara, H.; Shibata, H.; Prathumyot, W.; Waito, H.; Miyake, H. Arbsorption and distribution of Na+, Cl- and some others ions and physiological characteristic of sago palm under salt stress. Trop. Agric. Dev. 2008, 52, 7–16. [Google Scholar]
- Anugoolprasert, O.; Kinoshita, S.; Naito, H.; Shimizu, M.; Ehara, H. Effect of low pH on the growth, physiological characteristics and nutrient absorption of sago palm in hydroponic system. Plant Prod. Sci. 2012, 15, 125–131. [Google Scholar] [CrossRef]




| Growth Traits | Control | Waterlogging |
|---|---|---|
| Area of new developed leaflet (m2) | 0.35 ± 0.073 | 0.34 ± 0.032 |
| Leaf dry weight (g) | 9.71 ± 1.07 | 8.15 ± 0.59 |
| Petiole dry weight (g) | 20.95 ± 1.58 | 18.79 ± 0.91 |
| Plant height (cm) | 122 ± 6.64 | 117.63 ± 1.85 |
| Main root dry weight (g) | 17.35 ± 2.23 | 17.96 ± 2.23 |
| Secondary root dry weight (g) | 7.78 ± 1.20 | 11.07 ± 1.78 |
| Fine root dry weight (g) | 13.93 ± 3.09 | 18.99 ± 1.63 |
| Total root dry weight (g) | 39.06 ± 5.62 | 48.03 ± 5.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azhar, A.; Asano, K.; Sugiura, D.; Kano-Nakata, M.; Ehara, H. Waterlogged Conditions Influence the Nitrogen, Phosphorus, Potassium, and Sugar Distribution in Sago Palm (Metroxylon sagu Rottb.) at Seedling Stages. Plants 2022, 11, 710. https://doi.org/10.3390/plants11050710
Azhar A, Asano K, Sugiura D, Kano-Nakata M, Ehara H. Waterlogged Conditions Influence the Nitrogen, Phosphorus, Potassium, and Sugar Distribution in Sago Palm (Metroxylon sagu Rottb.) at Seedling Stages. Plants. 2022; 11(5):710. https://doi.org/10.3390/plants11050710
Chicago/Turabian StyleAzhar, Aidil, Koki Asano, Daisuke Sugiura, Mana Kano-Nakata, and Hiroshi Ehara. 2022. "Waterlogged Conditions Influence the Nitrogen, Phosphorus, Potassium, and Sugar Distribution in Sago Palm (Metroxylon sagu Rottb.) at Seedling Stages" Plants 11, no. 5: 710. https://doi.org/10.3390/plants11050710