Multiparent-Derived, Marker-Assisted Introgression Lines of the Elite Indian Rice Cultivar, ‘Krishna Hamsa’ Show Resistance against Bacterial Blight and Blast and Tolerance to Drought
Abstract
:1. Introduction
2. Results
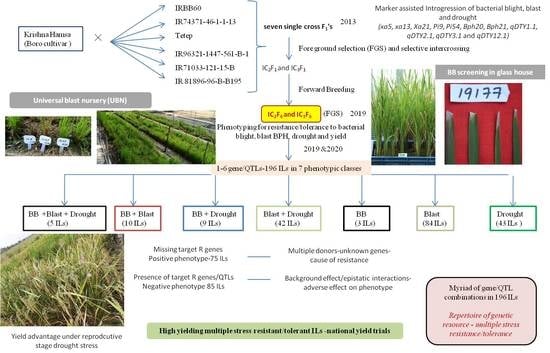
2.1. Selective InterCrossing to Combine Genes and QTLs
2.2. Gene/QTL Introgression vis-à-vis Phenotypic Response
2.2.1. Phenotypic Class-I Resistance/Tolerance to BB, Blast and Drought
2.2.2. Phenotypic Class-II Resistance/Tolerance to BB and Blast
2.2.3. Phenotypic Class-III Resistance/Tolerance to BB and Drought
2.2.4. Phenotypic Class-IV Resistance/Tolerance to Blast and Drought
2.2.5. Phenotypic Class-V Resistance/Tolerance to BB
2.2.6. Phenotypic Class-VI Resistance/Tolerance to Blast
2.2.7. Phenotypic Class-VII Tolerance to Drought
2.3. Background Selection
3. Discussion
4. Materials and Methods
4.1. Plant Material and Introgression Scheme
4.2. Genotyping
4.3. Phenotyping
4.3.1. Bacterial Leaf Blight Screening
4.3.2. Blast Screening
4.3.3. Brown Plant Hopper Screening
4.3.4. Yield Evaluation under Non-Stress and Drought Stress
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dixit, S.; Singh, U.M.; Singh, A.K.; Alam, S.; Venkateshwarlu, C.; Nachimuthu, V.V.; Yadav, S.; Abbai, R.; Selvaraj, R.; Devi, M.N.; et al. Marker Assisted Forward Breeding to Combine Multiple Biotic-Abiotic Stress Resistance/Tolerance in Rice. Rice 2020, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, R.M.; Vishnupriya, M.R.; Biradar, S.K.; Laha, G.S.; Reddy, G.A.; Rani, N.S.; Sarma, N.P.; Sonti, R.V. Marker assisted introgression of bacterial blight resistance in Samba Mahsuri, an elite indica rice variety. Euphytica 2008, 160, 411–422. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Nayak, D.K.; Mohanty, S.; Behera, L.; Barik, S.R.; Pandit, E.; Lenka, S.; Anandan, A. Pyramiding of three bacterial blight resistance genes for broad-spectrum resistance in deepwater rice variety, Jalmagna. Rice 2015, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ramayya, P.J.; Vinukonda, V.P.; Singh, U.M.; Alam, S.; Venkateshwarlu, C.; Vipparla, A.K.; Dixit, S.; Yadav, S.; Abbai, R.; Badri, J.; et al. Marker-assisted forward and backcross breeding for improvement of elite Indian rice variety Naveen for multiple biotic and abiotic stress tolerance. PLoS ONE 2021, 16, e0256721. [Google Scholar] [CrossRef]
- Singh, U.M.; Dixit, S.; Alam, S.; Yadav, S.; Prasanth, V.V.; Singh, A.K.; Venkateshwarlu, C.; Abbai, R.; Vipparla, A.K.; Badri, J.; et al. Marker-assisted forward breeding to develop a drought-, bacterial-leaf-blight-, and blast-resistant rice cultivar. Plant Genome 2021, e20170. [Google Scholar] [CrossRef]
- Swamy, H.K.M.; Anila, M.; Kale, R.R.; Rekha, G.; Bhadana, V.P.; Anantha, M.S.; Brajendra, P.; Balachiranjeevi, C.H.; Hajira, S.K.; Prasanna, B.L.; et al. Marker assisted improvement of low soil phosphorus tolerance in the bacterial blight resistant, fine-grain type rice variety, Improved Samba Mahsuri. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Yugander, A.; Sundaram, R.M.; Singh, K.; Ladhalakshmi, D.; Rao, L.V.S.; Madhav, M.S.; Badri, J.; Prasad, M.S.; Laha, G.S. Incorporation of the novel bacterial blight resistance gene Xa38 into the genetic background of elite rice variety Improved Samba Mahsuri. PLoS ONE 2018, 13, e0198260. [Google Scholar] [CrossRef]
- Kim, S.-M. Identification of novel recessive gene xa44(t) conferring resistance to bacterial blight races in rice by QTL linkage analysis using an SNP chip. Theor. Appl. Genet. 2018, 131, 2733–2743. [Google Scholar] [CrossRef] [Green Version]
- Neelam, K.; Mahajan, R.; Gupta, V.; Bhatia, D.; Gill, B.K.; Komal, R.; Lore, J.S.; Mangat, G.S.; Singh, K. High-resolution genetic mapping of a novel bacterial blight resistance gene xa-45(t) identified from Oryza glaberrima and transferred to Oryza sativa. Theor. Appl. Genet. 2019, 133, 689–705. [Google Scholar] [CrossRef]
- Suh, J.-P.; Jeung, J.-U.; Noh, T.-H.; Cho, Y.-C.; Park, S.-H.; Park, H.-S.; Shin, M.-S.; Kim, C.-K.; Jena, K.K. Development of breeding lines with three pyramided resistance genes that confer broad-spectrum bacterial blight resistance and their molecular analysis in rice. Rice 2013, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, R.M.; Madhav, M.S.; Balachandran, S.M.; Neeraja, C.N.; Mangrauthia, S.K.; Padmavathi, G. Marker-assisted selection for biotic stress resistance in rice. Dir. Rice Res. Tech. Bull. 2014, 79. [Google Scholar]
- Wang, S.; Liu, W.; Lu, D.; Lu, Z.; Wang, X.; Xue, J.; He, X. Distribution of Bacterial Blight Resistance Genes in the Main Cultivars and Application of Xa23 in Rice Breeding. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Yoshimura, A.; Nelson, R.; Mew, T.W.; Iwata, N. Tagging Genes for Bacterial Blight Resistance in Rice by RFLP and RAPD Markers. New Horizons Nitrogen Fixation 1993, 15, 250–253. [Google Scholar] [CrossRef]
- Zhang, G.; Angeles, E.R.; Abenes, M.L.P.; Huang, N.; Khush, G.S. RAPD and RFLP mapping of the bacterial blight resistance gene xa-13 in rice. Theor. Appl. Genet. 1996, 93, 65–70. [Google Scholar] [CrossRef]
- Ronald, P.C.; Albano, B.; Tabien, R.; Abenes, L.; Wu, K.-S.; McCouch, S.; Tanksley, S.D. Genetic and physical analysis of the rice bacterial blight disease resistance locus, Xa21. Mol. Genet. Genomics 1992, 236, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, N.; Angeles, E.R.; Domingo, J.; Magpantay, G.; Singh, S.; Zhang, G.; Kumaravadivel, N.; Bennett, J.; Khush, G.S. Pyramiding of bacterial blight resistance genes in rice: Marker-assisted selection using RFLP and PCR. Theor. Appl. Genet. 1997, 95, 313–320. [Google Scholar] [CrossRef]
- Singh, S.; Sidhu, J.S.; Huang, N.; Vikal, Y.; Li, Z.; Brar, D.S.; Dhaliwal, H.S.; Khush, G.S. Pyramiding three bacterial blight resistance genes (xa5, xa13 and Xa21) using marker-assisted selection into indica rice cultivar PR106. Theor. Appl. Genet. 2001, 102, 1011–1015. [Google Scholar] [CrossRef]
- ICAR-Indian Institute of Rice Research. Progress Report, 2020, Varietal Improvement; All India Coordinated Rice Improvement Project; Rajendranagar, Hyderabad-30, TS; ICAR-Indian Institute of Rice Research: New Delhi, India, 2021; Volume 1, p. 542.
- ICAR-Indian Institute of Rice Research. Progress Report, 2019, Varietal Improvement; India Coordinated Rice Improvement Project, ICAR-Indian Institute of Rice Research, Rajendranagar, Hyderabad-30, TS; ICAR-Indian Institute of Rice Research: New Delhi, India, 2020; Volume 1, p. 683.
- Babujee, L.; Gnanamanickam, S.S. Molecular tools for characterization of rice blast pathogen (Magnaporthe grisea) population and molecular marker-assisted breeding for disease resistance. Curr. Sci. 2000, 78, 248–257. [Google Scholar]
- Thulasinathan, T.; Kambale, R.; Ayyenar, B.; Manonmani, S.; Muthurajan, R. Evaluation of blast resistance genes Pi9 and Pi54 in rice against local isolates of Tamil Nadu. Electron. J. Plant Breed. 2020, 11, 1153–1158. [Google Scholar]
- Alam, S.; Imam, J.; Nitin, M.; Prasad, C.; Variar, M. Molecular Screening of Blast Resistance Gene Pi2 in Indian Rice Landraces (Oryza sativa L.) and its Verification by Virulence Analysis. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2017, 87, 67–72. [Google Scholar] [CrossRef]
- Rajashekara, H.; Ellur, R.K.; Khanna, A.P.U.R.V.A.; Nagarajan, M.; Gopalakrishnan, S.; Singh, A.K.; Sharma, P.; Sharma, T.R.; Singh, U.D. Inheritance of blast resistance and its allelic relationship with five major R genes in a rice landrace ‘Vanasurya’. Indian Phytopathol. 2014, 67, 365–369. [Google Scholar]
- Sharma, T.R.; Rai, A.K.; Gupta, S.K.; Vijayan, J.; Devanna, B.N.; Ray, S. Rice Blast Management Through Host-Plant Resistance: Retrospect and Prospects. Agric. Res. 2012, 1, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Ning, X.; Yunyu, W.; Aihong, L. Strategy for Use of Rice Blast Resistance Genes in Rice Molecular Breeding. Rice Sci. 2020, 27, 263–277. [Google Scholar] [CrossRef]
- Mackill, D.J.; Bonman, J.M. Inheritance of blast resistance in near-isogenic lines of rice. Phytopathology 1992, 82, 746–749. [Google Scholar] [CrossRef]
- Amante-Bordeos, A.; Sitch, L.A.; Nelson, R.; Dalmacio, R.D.; Oliva, N.P.; Aswidinnoor, H.; Leung, H. Transfer of bacterial blight and blast resistance from the tetraploid wild rice Oryza minuta to cultivated rice, Oryza sativa. Theor. Appl. Genet. 1992, 84, 345–354. [Google Scholar] [CrossRef]
- Sharma, T.R.; Shanker, P.; Singh, B.K.; Jana, T.K.; Madhav, M.S.; Gaikwad, K.; Plaha, P.; Rathour, R. Molecular Mapping of Rice Blast Resistance Gene Pi-kh in the Rice Variety Tetep. J. Plant Biochem. Biotechnol. 2005, 14, 127–133. [Google Scholar] [CrossRef]
- ICAR-Indian Institute of Rice Research. Progress Report, 2016, Varietal Improvement; All India Coordinated Rice Improvement Project ICAR-Indian Institute of Rice Research; Rajendranagar, Hyderabad-500 030, T.S; ICAR-Indian Institute of Rice Research: New Delhi, India, 2017; Volume 1.
- Eashkani, S.; Rafii, M.Y.; Eshabanimofrad, M.; Miah, G.; Esahebi, M.; Eazizi, P.; Tanweer, F.A.; Eakhtar, M.S.; Enasehi, A. Molecular Breeding Strategy and Challenges Towards Improvement of Blast Disease Resistance in Rice Crop. Front. Plant Sci. 2015, 6, 886. [Google Scholar] [CrossRef] [Green Version]
- Rahman, L.; Jiang, W.; Chu, S.H.; Qiao, Y.; Ham, T.-H.; Woo, M.-O.; Lee, J.; Khanam, M.S.; Chin, J.-H.; Jeung, J.-U.; et al. High-resolution mapping of two rice brown planthopper resistance genes, Bph20(t) and Bph21(t), originating from Oryza minuta. Theor. Appl. Genet. 2009, 119, 1237–1246. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, J.; Li, Z.; Liu, J.; Gao, G.; Zhang, Q.; Xiao, J.; He, Y. Evaluation and breeding application of six brown planthopper resistance genes in rice maintainer line Jin 23B. Rice 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Pandey, S.; Bhandari, H.; Sharan, R.; Naik, D.; Taunk, S.K.; Sastri, A.S.R.A.S. Economic Costs of Drought and Rainfed Rice Farmers’ Coping Mechanisms in Eastern India; Final Project Report; IRRI: Laguna, Philippines, 2005. [Google Scholar]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Mishra, K.K.; Vikram, P.; Yadaw, R.B.; Swamy, B.M.; Dixit, S.; Cruz, M.T.S.; Maturan, P.; Marker, S.; Kumar, A. qDTY12.1: A locus with a consistent effect on grain yield under drought in rice. BMC Genet. 2013, 14, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swamy, B.P.M.; Ahmed, H.U.; Henry, A.; Mauleon, R.; Dixit, S.; Vikram, P.; Tilatto, R.; Verulkar, S.B.; Perraju, P.; Mandal, N.P.; et al. Genetic, Physiological, and Gene Expression Analyses Reveal That Multiple QTL Enhance Yield of Rice Mega-Variety IR64 under Drought. PLoS ONE 2013, 8, e62795. [Google Scholar] [CrossRef]
- Venuprasad, R.; Dalid, C.O.; Del Valle, M.; Zhao, D.; Espiritu, M.; Cruz, M.T.S.; Amante, M.; Kumar, A.; Atlin, G.N. Identification and characterization of large-effect quantitative trait loci for grain yield under lowland drought stress in rice using bulk-segregant analysis. Theor. Appl. Genet. 2009, 120, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Venuprasad, R.; Bool, M.E.; Quiatchon, L.; Cruz, M.T.S.; Amante, M.; Atlin, G.N. A large-effect QTL for rice grain yield under upland drought stress on chromosome 1. Mol. Breed. 2011, 30, 535–547. [Google Scholar] [CrossRef]
- Yadaw, R.B.; Dixit, S.; Raman, A.; Mishra, K.K.; Vikram, P.; Swamy, B.M.; Cruz, M.T.S.; Maturan, P.T.; Pandey, M.; Kumar, A. A QTL for high grain yield under lowland drought in the background of popular rice variety Sabitri from Nepal. Field Crop. Res. 2013, 144, 281–287. [Google Scholar] [CrossRef]
- Shamsudin, N.A.A.; Swamy, B.P.M.; Ratnam, W.; Cruz, M.T.S.; Sandhu, N.; Raman, A.K.; Kumar, A. Pyramiding of drought yield QTLs into a high quality Malaysian rice cultivar MRQ74 improves yield under reproductive stage drought. Rice 2016, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, N.; Dixit, S.; Swamy, B.P.M.; Raman, A.; Kumar, S.; Singh, S.P.; Yadaw, R.B.; Singh, O.N.; Reddy, J.N.; Anandan, A.; et al. Marker Assisted Breeding to Develop Multiple Stress Tolerant Varieties for Flood and Drought Prone Areas. Rice 2019, 12, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Teh, S.L.; Ramirez, J.F.; Clark, M.; Gadoury, D.M.; Sun, Q.; Cadle-Davidson, L.; Luby, J.J. Genetic dissection of powdery mildew resistance in interspecific half-sib grapevine families using SNP-based maps. Mol. Breed. 2017, 37, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ramalingam, J.; Savitha, P.; Alagarasan, G.; Saraswathi, R.; Chandrababu, R. Functional Marker Assisted Improvement of Stable Cytoplasmic Male Sterile Lines of Rice for Bacterial Blight Resistance. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.I.; Ismail, S.I.; Oladosu, Y.; Kolapo, K.; Musa, I.; Halidu, J.; Muhammad, I.; Ahmed, M. Marker-Assisted Introgression of Multiple Resistance Genes Confers Broad Spectrum Resistance against Bacterial Leaf Blight and Blast Diseases in PUTRA-1 Rice Variety. Agronomy 2019, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Roychoudhury, A. Expression Profiling of Abiotic Stress-Inducible Genes in response to Multiple Stresses in Rice (Oryza sativaL.) Varieties with Contrasting Level of Stress Tolerance. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharoni, A.M.; Nuruzzaman, M.; Satoh, K.; Moumeni, A.; Attia, K.; Venuprasad, R.; Serraj, R.; Kumar, A.; Leung, H.; Islam, A.K.M.R.; et al. Comparative transcriptome analysis of AP2/EREBP gene family under normal and hormone treatments, and under two drought stresses in NILs setup by Aday Selection and IR64. Mol. Genet. Genom. 2011, 287, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidi, A.; Hajibarat, Z. Characterization of cis-elements in hormonal stress-responsive genes in Oryza sativa. Asia Pac. J. Mol. Biol. Biotechnol. 2019, 27, 95–102. [Google Scholar] [CrossRef]
- Sundaram, R.M.; Vishnupriya, M.R.; Laha, G.S.; Rani, N.S.; Rao, P.S.; Balachandran, S.M.; Reddy, G.A.; Sarma, N.P.; Sonti, R.V. Introduction of bacterial blight resistance into Triguna, a high yielding, mid-early duration rice variety. Biotechnol. J. 2009, 4, 400–407. [Google Scholar] [CrossRef]
- Xiao, C.; Hu, J.; Ao, Y.-T.; Cheng, M.-X.; Gao, G.-J.; Zhang, Q.-L.; He, G.-C.; He, Y.Q. Development and evaluation of near-isogenic lines for brown planthopper resistance in rice cv. 9311. Sci. Rep. 2016, 6, 38159. [Google Scholar] [CrossRef] [Green Version]
- Haque, A.; Rafii, M.Y.; Yusoff, M.M.; Ali, N.S.; Yusuff, O.; Datta, D.R.; Anisuzzaman, M.; Ikbal, M.F. Recent Advances in Rice Varietal Development for Durable Resistance to Biotic and Abiotic Stresses through Marker-Assisted Gene Pyramiding. Sustainability 2021, 13, 10806. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, S.; Ram, T.; Yadaw, R.B.; Mishra, K.K.; Mandal, N.P. Breeding high-yielding drought-tolerant rice: Genetic variations and conventional and molecular approaches. J. Exp. Bot. 2014, 65, 6265–6278. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sandhu, N.; Dixit, S.; Yadav, S.; Swamy, B.P.M.; Shamsudin, N.A.A. Marker-assisted selection strategy to pyramid two or more QTLs for quantitative trait-grain yield under drought. Rice 2018, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, A.; Jayaswal, P.; Yadav, N.; Singh, R.; Singh, Y.; Singh, B.; Singh, N.; Singh, S.; Sevanthi, A.; Rai, V.; et al. Genomics-assisted backcross breeding for infusing climate resilience in high-yielding green revolution varieties of rice. Indian J. Genet. Plant Breed. 2019, 79, 160–170. [Google Scholar] [CrossRef]
- Doyle, J. DNA Protocols for Plants. In Molecular Techniques in Taxonomy; Hewitt, G.M., Johnston, A.W.B., Young, J.P.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 283–293. [Google Scholar] [CrossRef]
- Standard Evaluation System (SES) for Rice, 5th ed.; IRRI: Los Baños, Philippines, 2013; Volume 5.
- Kalode, M.B.; Viswanathan, P.K.; Seshu, D.V. Standard test to characterize host plant resistance to brown planthopper in rice. Indian J. Plant Prot. 1975, 3, 204–206. [Google Scholar]
- Standard Evaluation System (SES) for Rice; IRRI: Los Baños, Philippines, 2002; p. 56.






| Gene/QTL Combination | ILs with BB, BL Scores and % YA over RP under RDS | No. of ILs |
|---|---|---|
| Phenotypic class I: Resistance/tolerance to BB, blast and drought (three traits) | ||
| Xa21+Pi9+qDTY2.1+qDTY3.1 | 19196 (5, 4, +120.27%) | 1 |
| xa5+Xa21+qDTY3.1 | 19174 (5, 4, +82.28%), 19193 (5, 4, +68.23%) | 2 |
| qDTY2.1 | 19246 (1, 1, +180.43%), 19247 (1, 4, +131.13%) | 2 |
| Phenotypic class II: Resistance/tolerance to BB and blast (two traits) | ||
| Pi54+Bph20+Bph21 | 19030 (1,1) | 1 |
| xa5+Bph20+Bph21 | 19007 (5, 3), 19020 (5, 1), 19025 (5, 1), 19039 (5, 4), 19406 (5,1) | 5 |
| xa5+Pi9 | 19019 (3,1), 19471 (3,1) | 2 |
| xa5+Xa21+Bph20+Bph21 | 19031 (1,4) | 1 |
| xa13+Xa21+Pi54 | 19378 (1,1) | 1 |
| Phenotypic class III: Resistance/tolerance to BB and drought (two traits) | ||
| Pi9+qDTY2.1 | 19232 (1, +127.06%), 19238 (1, +73.23%), 19239 (1, +59.66%), 19240 (1, +125.52%), 19244 (1, +30.72%) | 5 |
| xa5+Pi9+qDTY2.1 | 19233 (1, +232.44%) | 1 |
| qDTY2.1 | 19241 (1, +60.12%), | 1 |
| xa5+qDTY2.1 | 19245 (1, +230.63%), 19248 (1, +135.20%) | 2 |
| Phenotypic class IV: Resistance/tolerance to blast and drought (two traits) | ||
| xa5+Xa21+Pi9+qDTY12.1+qDTY2.1 | 19208 (1, +72.78%) | 1 |
| xa5+Xa21+Pi9+qDTY12.1+qDTY3.1 | 19182 (3, +46.09%) | 1 |
| xa5+Xa21+Pi9+qDTY2.1+qDTY3.1 | 19194 (4, +101.56%), 19195 (4, +153.65%), 19197 (4, +55.14%), 19198 (4, +260.03%), 19200 (4, +98.11%), 19201 (4, +198.97%), 19267 (3, +164.60%) | 7 |
| xa5+Xa21+qDTY1.1+qDTY2.1+qDTY12.1 | 19177 (5, +55.14%) | 1 |
| Xa21+Pi9+qDTY12.1+qDTY3.1 | 19185 (3, +14.88%) | 1 |
| Xa21+Pi9+qDTY2.1+qDTY3.1 | 19199 (4, +167.76%) | 1 |
| Xa21+qDTY12.1+qDTY2.1+qDTY3.1 | 19189 (4, +82.73%), 19191 (3, +80.47%), 19192 (4, +133.39%) | 3 |
| xa5+Xa21+Pi9+qDTY12.1 | 19263 (4, +129.32%) | 1 |
| xa5+Xa21+Pi9+qDTY2.1 | 19206 (3, +42.02%) | 1 |
| qDTY12.1+qDTY2.1+qDTY3.1 | 19190 (1, +68.75%) | 1 |
| Xa21+Pi9+qDTY2.1 | 19214 (2, +113.49%), 19215 (2, +211.18%), 19249 (4, +140.17%) | 3 |
| xa21+pi9+qDTY3.1 | 19237 (4, +62.83%), 19262 (4, +178.62%), 19264 (1, +155.10%), 19271 (4, +52.43%) | 4 |
| Xa21+qDTY12.1+qDTY2.1 | 19250 (4, +98.56%) | 1 |
| Xa21+qDTY2.1+qDTY3.1 | 19261 (4, +154.19%) | 1 |
| xa5+Pi9+qDTY2.1 | 19203 (3, +55.59%) | 1 |
| xa5+qDTY2.1+qDTY3.1 | 19211 (3, +134.75%) | 1 |
| xa5+Xa21+qDTY3.1 | 19176 (4, +68.71%) | 1 |
| Pi9+qDTY3.1 | 19271 (4, +52.43%), 19274 (4, +202.59%), 19279 (3, +148.31%) | 3 |
| qDTY12.1+qDTY3.1 | 19183 (4, +113.94%) | 1 |
| qDTY2.1+qDTY3.1 | 19253 (1, +126.15%), 19254 (4, +222.04%) | 2 |
| Xa21+qDTY2.1 | 19268 (4, +188.57%) | 1 |
| Xa21+qDTY3.1 | 19221 (4, +114.39%), 19275 (4, +143.34%) | 2 |
| xa5+qDTY2.1 | 19205 (4, +16.69%) | 1 |
| qDTY12.1 | 19178 (5, +256.87%) | 1 |
| qDTY3.1 | 19181 (3, +163.69%) | 1 |
| Gene/QTL Combination | IL with Mean BB/Blast Score | No. of ILs |
|---|---|---|
| Phenotypic class V: resistance/tolerance to bacterial blight | ||
| xa5+Xa21+Pi54 | 19460 (1) | 1 |
| xa5+Xa21+Pi54 | 19379 (1) | 1 |
| Bph20 | 19046 (1) | 1 |
| Phenotypic class VI: resistance/tolerance to blast | ||
| Xa21+Pi9+qDTY12.1+qDTY2.1+qDTY3.1 | 19188 (1) | 1 |
| xa5+Pi9+pi54+Bph20+Bph21 | 19013 (1) | 1 |
| Xa21+Pi9+Bph20+Bph21 | 19392 (4) | 1 |
| xa5+Pi9+Bph20+Bph21 | 19464 (4) | 1 |
| Xa21+Pi9+qDTY12.1+qDTY2.1 | 19186 (1) | 1 |
| xa5+Pi9+qDTY2.1+qDTY3.1 | 19172 (1) | 1 |
| xa5+Xa21+Pi9+qDTY2.1 | 19207 (1) | 1 |
| xa21+pi9+qDTY3.1 | 19167 (1) | 1 |
| Bph20+Bph21+Pi9 | 19386 (1), 19387 (1) | 2 |
| Pi54+Bph20+Bph21 | 19001 (1), 19014 (2), 19015 (2), 19033 (1), 19035 (4), 19037 (4), 19038 (4), 19043 (4), 19044 (1), 19045 (1) | 10 |
| xa5+Pi9+Bph20+Bph21 | 19022 (1) | 1 |
| Pi9+Bph20+Bph21 | 19006 (4) | 1 |
| xa5+Pi9+qDTY2.1 | 19204 (4) | 1 |
| Bph20+Bph21 | 19004 (4), 19005 (3), 19008 (1), 19021 (1), 19026 (1), 19028 (4), 19032 (3), 19040 (3), 19394 (1), 19405 (4), | 10 |
| xa5+Bph20+Bph21 | 19023 (2), 19024 (1) | 2 |
| xa5+qDTY2.1 | 19202 (1) | 1 |
| Pi9+qDTY3.1 | 19270 (1) | 1 |
| Pi9+qDTY3.1 | 19272 (1) | 1 |
| Pi9+Bph20 | 19052 (4) | 1 |
| Pi9+Pi54 | 19055 (4) | 1 |
| xa13+Pi9 | 19396 (2), 19401 (3), 19402 (3), 19411 (2), 19413 (4), 19467 (5), 19470 (3) | 7 |
| Xa21+Pi54 | 19459 (5) | 1 |
| xa5+Pi9 | 19399 (4), 19407 (3), 19408 (3), 19409 (2), 19461 (3) | 5 |
| Bph20 | 19048 (4), 19049 (4), 19053 (3), 19054 (4), 19056 (3) | 5 |
| Pi54 | 19027 (3), 19034 (4), 19042 (4), 19050 (4) | 4 |
| xa5 +Pi9 | 19018 (3) | 1 |
| xa5+Pi9 | 19420 (2), 19421 (2) | 2 |
| xa13 | 19389 (4), 19403 (4), 19410 (2), 19412 (1), 19415 (4), 19416 (3), 19462 (4), 19463 (3), 19465 (4) | 9 |
| Xa21 | 19180 (3), 19243 (4), 19466 (4), 19468 (4) | 4 |
| xa5 | 19397 (1), 19417 (4) | 2 |
| xa5+xa13 | 19469 (4) | 1 |
| xa5+Xa21+Pi9 | 19210 (3) | 1 |
| No genes | 19175 (4), 19400 (1) | 2 |
| Gene/QTL Combination | ILs with % YA over RP under RDS | No. of ILs |
|---|---|---|
| xa5+Xa21+Pi9+qDTY12.1+qDTY3.1. | 19280 (+204.4%), 19281 (+149.22%) | 2 |
| xa5+Xa21+qDTY12.1+qDTY2.1+qDTY3.1 | 19184 (+49.26%) | 1 |
| Xa21+Pi9+qDTY2.1+qDTY3.1 | 19257 (+79.11%), 19258 (+115.75%), 19259 (+128.87%), 19266 (+146.05%) | 4 |
| xa5+Pi9+qDTY12.1+qDTY2.1 | 19209 (+87.71%) | 1 |
| xa5+Xa21+Pi9+qDTY12.1 | 19266 (+146.05%) | 1 |
| Xa21+Pi9+qDTY2.1 | 19222 (+164.6%), 19223 (+16.86%), 19224 (+189.93%), 19225 (+184.5%), 19231 (+215.25%), 19234 (+203.04%), 19255 (+23.93%) | 7 |
| Xa21+pi9+qDTY3.1 | 19170 (+134.29%) | 1 |
| xa5+Pi9+qDTY2.1 | 19217 (+82.73%), 19218 (+91.78%), 19219 (+172.29%), 19220 (+32.52%), 19229 (+32.52%) | 5 |
| xa5+Xa21+qDTY12.1 | 19173 (+154.65%) | 1 |
| Pi9+qDTY2.1 | 19213 (+101.73%), 19226 (+275.86%), 19227 (+63.73%), 19228 (+103.08%), 19230 (+23.48%), 19242 (+146.5%) | 6 |
| pi9+qDTY3.1 | 19168 (+26.64%), 19171 (+28.91%), 19277 (+184.95%), 19278 (+243.75%) | 4 |
| qDTY2.1+qDTY3.1 | 19166 (+110.32%) | 2 |
| Xa21+qDTY2.1 | 19235 (+161.88%), 19251 (+216.61%), 19269 (+153.65%) | 3 |
| xa5+qDTY2.1 | 19236 (+112.58%) | 1 |
| qDTY12.1 | 19187 (+43.38%) | 1 |
| qDTY2.1 | 19252 (+134.75%) | 1 |
| qDTY3.1 | 19169 (+83.63%) | 1 |
| xa5+Xa21+qDTY2.1 | 19216 (+134.75%) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badri, J.; Lakshmidevi, G.; JaiVidhya, L.R.K.; Prasad, M.S.; Laha, G.S.; Lakshmi, V.J.; Isetty, S.R.; Padmashree, R.; Balakrishnan, D.; Varanasi, Y.V.P.; et al. Multiparent-Derived, Marker-Assisted Introgression Lines of the Elite Indian Rice Cultivar, ‘Krishna Hamsa’ Show Resistance against Bacterial Blight and Blast and Tolerance to Drought. Plants 2022, 11, 622. https://doi.org/10.3390/plants11050622
Badri J, Lakshmidevi G, JaiVidhya LRK, Prasad MS, Laha GS, Lakshmi VJ, Isetty SR, Padmashree R, Balakrishnan D, Varanasi YVP, et al. Multiparent-Derived, Marker-Assisted Introgression Lines of the Elite Indian Rice Cultivar, ‘Krishna Hamsa’ Show Resistance against Bacterial Blight and Blast and Tolerance to Drought. Plants. 2022; 11(5):622. https://doi.org/10.3390/plants11050622
Chicago/Turabian StyleBadri, Jyothi, Gandhudi Lakshmidevi, L. R. K. JaiVidhya, Madamsetty Srinivasa Prasad, Gouri Shankar Laha, Vattikutti Jhansi Lakshmi, Subhakara Rao Isetty, Revadi Padmashree, Divya Balakrishnan, Yasaswini Vishnu Priya Varanasi, and et al. 2022. "Multiparent-Derived, Marker-Assisted Introgression Lines of the Elite Indian Rice Cultivar, ‘Krishna Hamsa’ Show Resistance against Bacterial Blight and Blast and Tolerance to Drought" Plants 11, no. 5: 622. https://doi.org/10.3390/plants11050622