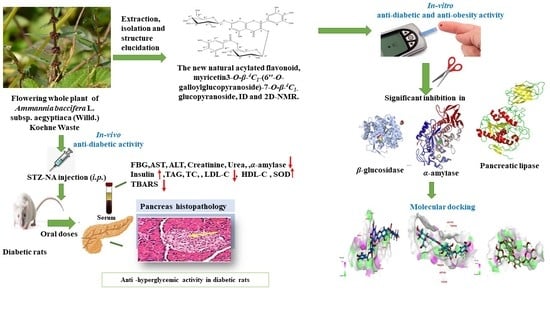
Antidiabetic Activity and In Silico Molecular Docking of Polyphenols from Ammannia baccifera L. subsp. Aegyptiaca (Willd.) Koehne Waste: Structure Elucidation of Undescribed Acylated Flavonol Diglucoside
Abstract
:1. Introduction
2. Results
2.1. Total Phenolic and Flavonoid Contents
2.2. Identification of Polyphenols from AEEE
2.3. Molecular Docking
2.3.1. Interactions Analysis with α-Amylase
2.3.2. Interactions Analysis with β-Glucosidase
2.3.3. Interactions Analysis with Pancreatic Lipase
2.4. In Vitro Studies
2.4.1. DPPH Assay
2.4.2. ORAC Assay
2.4.3. Reducing Power Assay
2.4.4. In Vitro Enzyme Assays
2.5. In Vivo Assays
2.5.1. Acute Oral Toxicity
2.5.2. Effect of AEEE on Body Weight
2.5.3. Effect of AEEE on Liver and Kidney Function Markers
2.5.4. Effect of AEEE on Serum Blood Glucose, Insulin and α-Amylase
2.5.5. Effect of AEEE on Serum Lipid Profile
2.5.6. Effect of AEEE on Oxidative Stress Markers of the Pancreas
2.5.7. Effect of AEEE on Pancreas Histopathological Examination
3. Discussion
4. Materials and Methods
4.1. General
4.2. Plant Materials
4.3. Preparation of AEEE
4.4. Estimation of Total Phenolic and Flavonoid Contents
4.5. Isolation and Identification of Phenolics (1–5)
Myricetin 3-O-β-4C1-(6”-O-Galloyl Glucopyranoside) 7-O-β-4C1 Glucopyranoside, MGGG, New Compound 1
4.6. Molecular Modeling
4.7. In Vitro Studies
4.7.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
4.7.2. Oxygen Radical Absorbance Capacity (ORAC Assay)
4.7.3. Reducing Power Assay
4.7.4. α-Amylase Inhibition
4.7.5. β-Glucosidase Inhibition
4.7.6. Pancreatic Lipase Inhibition
4.8. In Vivo Studies
4.8.1. Experimental Animals
4.8.2. Acute Oral Toxicity
4.8.3. Induction of Diabetes
4.8.4. Experimental Design
4.8.5. Blood and Tissue Sampling
4.8.6. Assay of Biochemical Markers
Determination of Liver and Kidney Functions Markers
Determination of Insulin and α-Amylase Activity
Measurement of Serum Lipid Profile
Determination of Oxidative Stress Markers in Pancreatic Tissue
4.8.7. Histopathological Investigation
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, S.; Cutter, J.; Tan, C.E.; Chew, S.K.; Tai, E.S. Associations of diabetes mellitus and ethnicity with mortality in a multiethnic Asian population: Data from the 1992 Singapore National Health Survey. Am. J. Epidemiol. 2003, 158, 543–552. [Google Scholar] [CrossRef]
- Florencia, A.; Alex, B. IDF Diabetes Atlas, 6th ed.; International Diabetes Federation: Brussels, Belgium, 2014; pp. 1–160. [Google Scholar]
- Patel, D.; Kumar, R.; Prasad, S.; Sairam, K.; Hemalatha, S. Anti-diabetic and in vitro antioxidant potential of Hybanthus enneaspermus (Linn) F. Muell in streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2011, 1, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can. J. Diabetes 2018, 42, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Overview of food products and dietary constituents with antidiabetic properties and their putative mechanisms of action: A natural approach to complement pharmacotherapy in the management of diabetes. Mol. Nutr. Food Res. 2014, 58, 61–78. [Google Scholar] [CrossRef]
- Eckel, R.; Kahn, S.; Ferrannini, E.; Goldfine, A.; Nathan, D.; Schwartz, M.; Smith, R.; Smith, S. Obesity and Type 2 Diabetes: What Can Be 82 Unified and What Needs to Be Individualized? Diabetes Care 2011, 34, 1424–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Clark, M. Textbook of Clinical Medicine, 8th ed.; Saunders: London, UK, 2002. [Google Scholar]
- Bastaki, S. Review-diabetes milletus and its treatment. Int. J. Diabetes Metab. 2005, 13, 111–134. [Google Scholar] [CrossRef]
- Wais, M.; Nazish, I.; Samad, A.; Beg, S.; Abusufyan, S.; Ajaj, S.A.; Aqil, M. Herbal drugs for diabetic treatment: An updated review of patents. Recent Pat. Anti-Infect. Drug Discov. 2012, 7, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Carek, P.J.; Dickerson, L.M. Current concepts in the pharmacological management of obesity. Drugs 1999, 57, 883–904. [Google Scholar] [CrossRef]
- Yang, H.; Jin, X.; Kei Lam, C.W.; Yan, S.K. Oxidative stress and diabetes mellitus. Clin. Chem. Lab. Med. 2011, 49, 1773–1782. [Google Scholar] [CrossRef]
- Savu, O.; Ionescu-Tirgoviste, C.; Atanasiu, V.; Gaman, L.; Papacocea, R.; Stoian, I. Increase in total antioxidant capacity of plasma despite high levels of oxidative stress in uncomplicated type 2 diabetes mellitus. J. Int. Med. Res. 2012, 40, 709–716. [Google Scholar] [CrossRef]
- Patel, D.K.; Kumar, R.; Laloo, D.; Hemalatha, S. Diabetes mellitus: An overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac. J. Trop. Biomed. 2012, 2, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Sekhon-Loodu, S.; Rupasinghe, H.P.V. Evaluation of Antioxidant, Antidiabetic and Antiobesity Potential of Selected Traditional Medicinal Plants. Front. Nutr. 2019, 6, 53. [Google Scholar] [CrossRef]
- Nawwar, M.; Ayoub, N.; El-Raey, M.; Zaghloul, S.; Hashem, A.; Mostafa, E.; Eldahshan, O.; Lindequist, U.; Linscheid, M.W. Acylated flavonol diglucosides from Ammania auriculata. Z. Für Nat. C J. Biosci. 2015, 70, 39–43. [Google Scholar] [CrossRef]
- Boulos, L. Flora of Egypt. In Geraniaceae-Boraginaceae; Al Hadara Publ.: Cairo, Egypt, 2000; Volume 2. [Google Scholar]
- Tackholm, V. Students’ Flora of Egypt, 2nd ed.; Cairo University Press: Cairo, Egypt, 1974; p. 423. [Google Scholar]
- Patel, B.; Kori, M. Antidiabetic Effect of Ammania baccifera Linn leaf on Streptozotocin Induced Diabetes in Male Albino Wistar Rats. Res. J. Pharm. Technol. 2018, 11, 4773–4780. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, Y.; Lu, H.; Wang, X.; Jin, N. Comparative analysis of the bonding modes between two antidiabetic drugs with beta-glucosidases from different species. Indian J. Pharm. Sci. 2016, 78, 512–524. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Jia, Y.; Ma, Y.; Cheng, G.; Cai, S. Phenolic composition, antioxidant properties, and inhibition toward digestive enzymes with molecular docking analysis of different fractions from Prinsepia utilis royle fruits. Molecules 2018, 23, 3373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhong, C.H.; Riyaphan, J.; Lin, S.H.; Chia, Y.C.; Weng, C.F. Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. Biofactors 2015, 41, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Dehaghani, Z.A.; Asghari, G.; Dinani, M.S. Isolation and identification of nicotiflorin and narcissin from the aerial parts of Peucedanum aucheri Boiss. J. Agric. Sci. Technol. A 2017, 7, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Nawwar, M.; Ayoub, N.; Hussein, S.; Hashim, A.; El-Sharawy, R.; Wende, K.; Harms, M.; Lindequist, U. A flavonol triglycoside and investigation of the antioxidant and cell stimulating activities of Annona muricata Linn. Arch. Pharm. Res. 2012, 35, 761–767. [Google Scholar] [CrossRef]
- Nawwar, M.A.; Youb, N.A.; El-Raey, M.A.; Zaghloul, S.S.; Hashem, A.M.; Mostafa, E.S.; Eldahshan, O.; Werner, V.; Becker, A.; Haertel, B.; et al. Polyphenols in Ammania auriculata: Structures, antioxidative activity and cytotoxicity. Pharmazie 2014, 69, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Baharudin, M.S.; Ismail, N.H.; Imran, S.; Khan, M.N.; Rahim, F.; Selvaraj, M.; Chigurupati, S.; Nawaz, M.; Qureshi, F.; et al. Synthesis, α-amylase inhibitory potential and molecular docking study of indole derivatives. Bioorg. Chem. 2018, 80, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, J.; Hayashi, Y.; Baba, Y.; Okino, N.; Kimura, M.; Ito, M.; Kakuta, Y. Crystal structure of the covalent intermediate of human cytosolic beta-glucosidase. Biochem. Biophys. Res. Commun. 2008, 374, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Eydoux, C.; Spinelli, S.; Davis, T.L.; Walker, J.R.; Seitova, A.; Dhe-Paganon, S.; De Caro, A.; Cambillau, C.; Carriere, F. Structure of human pancreatic lipase-related protein 2 with the lid in an open conformation. Biochemistry 2008, 47, 9553–9564. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Karan, T.K.; Kar, B.; Bhattacharya, S.; Ghosh, A.K.; Kumar, R.S.; Haldar, P.K. Hepatoprotective activity of Terminalia arjuna leaf against paracetamol-induced liver damage in rats. Asian Pac. J. Chem. 2011, 23, 1739. [Google Scholar] [CrossRef] [Green Version]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Mishra, A.; Pandey, A.K. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement. Altern. Med. 2013, 13, 120. [Google Scholar] [CrossRef] [Green Version]
- Teissedre, P.L.; Frankel, E.N.; Waterhouse, A.L.; Peleg, H.; German, J.B. Inhibition ofIn vitrohuman LDL oxidation by phenolic antioxidants from grapes and wines. J. Sci. Food Agric. 1996, 70, 55–61. [Google Scholar] [CrossRef]
- Sarian, M.N.; Ahmed, Q.U.; Mat So’ad, S.Z.; Alhassan, A.M.; Murugesu, S.; Perumal, V.; Syed Mohamad, S.N.A.; Khatib, A.; Latip, J. Antioxidant and antidiabetic effects of flavonoids: A structure-activity relationship based study. Biomed. Res. Int. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y. Minireview: Therapeutic potential of myricetin in diabetes mellitus. Food Sci. Hum. Wellness 2012, 1, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.L.; Rusten, A.; Bugge, M.S.; Malterud, K.E.; Diallo, D.; Paulsen, B.S.; Wangensteen, H. Flavonoids, gallotannins and ellagitannins in Syzygium guineense and the traditional use among Malian healers. J. Ethnopharmacol. 2016, 192, 450–458. [Google Scholar] [CrossRef]
- Ceriello, A. Oxidative stress and glycemic regulation. Metabolism 2000, 49, 27–29. [Google Scholar] [CrossRef]
- Hassan, S.K.; El-Sammad, N.M.; Mousa, A.M.; Mohammed, M.H.; Farrag, A.H.; Hashim, A.N.; Lindequist, U.; Nawwar, M.A.; Werner, V. Hypoglycemic and antioxidant activities of Caesalpinia ferrea Martius leaf extract in streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2015, 5, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as α-Amylase and α-Glucosidase Inhibitors and their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini-Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Spínola, V.; Llorent-Martínez, E.J.; Castilho, P.C. Inhibition of α-amylase, α-glucosidase and pancreatic lipase by phenolic compounds of Rumex maderensis (Madeira sorrel). Influence of simulated gastrointestinal digestion on hyperglycaemia-related damage linked with aldose reductase activity and protein glycation. LWT 2020, 118, 108727. [Google Scholar] [CrossRef]
- Parizadeh, H.; Garampalli, R.H. Evaluation of Some Lichen Extracts for β-Glucosidase Inhibitory as a Possible Source of Herbal Anti-diabetic Drugs. Am. J. Biochem. Biotechnol. 2016, 6, 46–50. [Google Scholar] [CrossRef]
- Sánchez, M.A.; García, S.K.; May, P.F.; Pea, L.M.R. Evaluation of biological activity of crude extracts from plants used in Yucatecan traditional medicine Part I. Antioxidant, antimicrobial and β-glucosidase inhibitory activities. Phytomedicine 2001, 101, 633–649. [Google Scholar] [CrossRef]
- Mogale, M.; Lebelo, S.L.; Thovhogi, N.; De Freitas, A.; Shai, L. α-Amylase and α-glucosidase inhibitory effects of Sclerocarya birrea [(A. Rich.) Hochst.] subspecies caffra (Sond) Kokwaro (Anacardiaceae) stem-bark extracts. Afr. J. biotechnol. 2011, 10, 15033–15039. [Google Scholar] [CrossRef]
- Lowe, M.E. Pancreatic triglyceride lipase and colipase: Insights into dietary fat digestion. Gastroenterology 1994, 107, 1524–1536. [Google Scholar] [CrossRef]
- Schnee, D.M.; Zaiken, K.; McCloskey, W.W. An update on pharmacological treatment of obesity. Curr. Med. Res. Opin. 2006, 22, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Birari, R.; Bhutani, K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Ayeni, P.O.; Omojokun, O.S.; Bello, F. Comparative effect of quercetin and rutin on α-amylase, α-glucosidase, and some pro-oxidant-induced lipid peroxidation in rat pancreas. Comp. Clin. Pathol. 2015, 24, 1103–1110. [Google Scholar] [CrossRef]
- Lee, S.-S.; Lin, H.-C.; Chen, C.-K. Acylated flavonol monorhamnosides, α-glucosidase inhibitors, from Machilus philippinensis. Phytochemistry 2008, 69, 2347–2353. [Google Scholar] [CrossRef]
- Pepato, M.; Mori, D.; Baviera, A.; Harami, J.; Vendramini, R.; Brunetti, I. Fruit of the jambolan tree (Eugenia jambolana Lam.) and experimental diabetes. J. Ethnopharmacol. 2005, 96, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Balomajumder, C.; Roy, P. Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food Chem. Toxicol. 2008, 46, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, N.M.; Radwan, R.A.; Ali, S.M.; Saad, H.H. Molecular networking aided metabolomic profiling of beet leaves using three extraction solvents and in relation to its anti-obesity effects. J. Adv. Res. 2020, 24, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, R.F.; Ezzat, S.M.; Ogaly, H.A.; Abd-Elsalam, R.M.; Hessin, A.F.; Fekry, M.I.; Mansour, D.F.; Mohamed, S.O. Ficus deltoidea extract down-regulates protein tyrosine phosphatase 1B expression in a rat model of type 2 diabetes mellitus: A new insight into its antidiabetic mechanism. J. Nutr. Sci. 2020, 9, e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafa, E.S.; Nawwar, M.A.; Mostafa, D.A.; Ragab, M.F.; Swilam, N. Karafsin, a unique mono-acylated flavonoid apiofurnoside from the leaves of Apium graveolens var. secalinum Alef: In vitro and in vivo anti-inflammatory assessment. Ind. Crops. Prod. 2020, 158, 112901. [Google Scholar] [CrossRef]
- Al-Karmalawy, A.A.; Eissa, I.H. Molecular docking and dynamics simulations reveal the potential of anti-HCV drugs to inhibit COVID-19 main protease. Pharm. Sci. 2021, 10. [Google Scholar] [CrossRef]
- Maurus, R.; Begum, A.; Williams, L.K.; Fredriksen, J.R.; Zhang, R.; Withers, S.G.; Brayer, G.D. Alternative catalytic anions differentially modulate human alpha-amylase activity and specificity. Biochemistry 2008, 47, 3332–3344. [Google Scholar] [CrossRef]
- Nasser, A.A.; Eissa, I.H.; Oun, M.R.; El-Zahabi, M.A.; Taghour, M.S.; Belal, A.; Saleh, A.M.; Mehany, A.B.; Luesch, H.; Mostafa, A.E. Discovery of new pyrimidine-5-carbonitrile derivatives as anticancer agents targeting EGFR WT and EGFR T790M. Org. Biomol. Chem. 2020, 18, 7608–7634. [Google Scholar] [CrossRef]
- Antony, P.; Vijayan, R. Identification of novel aldose reductase inhibitors from spices: A molecular docking and simulation study. PLoS ONE 2015, 10, e0138186. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lucas-Abellán, C.; Mercader-Ros, M.; Zafrilla, M.; Fortea, M.; Gabaldón, J.; Núñez-Delicado, E. ORAC-fluorescein assay to determine the oxygen radical absorbance capacity of resveratrol complexed in cyclodextrins. J. Agric. Food Chem. 2008, 56, 2254–2259. [Google Scholar] [CrossRef] [PubMed]
- Deshavath, N.N.; Mukherjee, G.; Goud, V.V.; Veeranki, V.D.; Sastri, C.V. Pitfalls in the 3, 5-dinitrosalicylic acid (DNS) assay for the reducing sugars: Interference of furfural and 5-hydroxymethylfurfural. Int. J. Biol. Macromol. 2020, 156, 180–185. [Google Scholar] [CrossRef]
- Grover, A.K.; Macmurchie, D.D.; Cushley, R.J. Studies on almond emulsin beta-D-glucosidase. I. Isolation and characterization of a bifunctional isozyme. Biochim. Biophys. Acta 1977, 482, 98–108. [Google Scholar] [CrossRef]
- Shoieb, S.M.; Esmat, A.; Khalifa, A.E.; Abdel-Naim, A.B. Chrysin attenuates testosterone-induced benign prostate hyperplasia in rats. Food Chem. Toxicol. 2018, 111, 650–659. [Google Scholar] [CrossRef]
- Hassan, S.K.; El-Sammad, N.M.; Abdel-Halim, A.H.; Mousa, A.M.; Khalil, W.K.B.; Anwar, N. Flavonoids-rich Extract of Beta vulgaris Subsp cicla L. var. Flavescens Leaf, a Promising Protector Against Gentamicin-induced Nephrotoxicity and Hepatotoxicity in Rats. Int. J. Pharmacol. 2018, 14, 652–666. [Google Scholar] [CrossRef]
- Fukuyama, N.; Homma, K.; Wakana, N.; Kudo, K.; Suyama, A.; Ohazama, H.; Tsuji, C.; Ishiwata, K.; Eguchi, Y.; Nakazawa, H.; et al. Validation of the Friedewald Equation for Evaluation of Plasma LDL-Cholesterol. J. Clin. Biochem. Nutr. 2007, 43, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minami, M.; Yoshikawa, H. A simplified assay method of superoxide dismutase activity for clinical use. Clin. Chim. Acta 1979, 92, 337–342. [Google Scholar] [CrossRef]
- Papastergiadis, A.; Mubiru, E.; Van Langenhove, H.; De Meulenaer, B. Malondialdehyde measurement in oxidized foods: Evaluation of the spectrophotometric thiobarbituric acid reactive substances (TBARS) test in various foods. J. Agric. Food Chem. 2012, 60, 9589–9594. [Google Scholar] [CrossRef] [PubMed]









| Ligand | RMSD Value (Å) | Docking Score (kcal/mol) | Interactions and Residues | Distance (Å) |
|---|---|---|---|---|
| Acarbose | 1.99 | −8.131 |
His305 His201 Asp300 | 2.42 2.67 1.97 2.37 1.83 1.97 |
His101 | - | |||
| Compound 1 | 1.98 | −8.99 |
Asp197
Trp59 Ile235 Lleu162 | 2.84 2.17 2.31 - |
| Compound 2 | 1.94 | −7.07 |
Asp197 Asp300 Gln63 Thr163 | 2.45 2.24 1.97 2.15 3.08 2.69 |
| - | |||
| Compound 3 | 1.69 | −6.43 |
Asp197 Thr163
| 2.72 1.83 2.11 - |
| Compound 4 | 2.39 | −6.47 |
Asp300 Gln63 | 2.98 2.46 2.54 2.23 4.85 |
| - | |||
| Compound 5 | 1.88 | −6.50 |
Gln63 Tyr151 Glu233 His305 Tyr163 | 2.01 3.06 2.85 2.52 2.33 1.83 |
| - |
| Ligand | RMSD Value (Å) | Docking Score (kcal/mol) | Interactions and Residues | Distance (Å) |
|---|---|---|---|---|
| Acarbose | 1.77 | −5.04 |
Asn319 Gln271 Ser270 Glu331 | 2.68 2.50 2.82 2.68 2.36 |
| Compound 1 | 1.88 | −6.43 |
Glu318 Asn319 Met275 Gln279 Lys278 Gln271
| 2.12 2.41 2.28 2.65 2.94 2.90 2.91 2.78 - |
| Compound 2 | 2.03 | −5.28 |
Glu331 Asn319 Lys278 | 1.92 2.22 2.73 2.10 2.46 2.74 2.02 |
| Compound 3 | 2.56 | −5.42 |
Lys278 Gln271 Tyr316 Glu241 Arg245 | 2.10 2.50 2.04 5.02 1.93 2.24 3.07 3.07 |
| Compound 4 | 1.68 | −5.36 |
Lys278 | 5.26 3.34 2.81 |
| - | |||
| Compound 5 | 1.18 | −5.70 |
Ser238 Lys315 Gln271 Lys278 | 1.96 1.98 2.41 2.78 2.22 2.85 2.29 |
| - |
| Ligand | RMSD Value (Å) | Docking Score (kcal/mol) | Interactions and Residues | Distance (Å) |
|---|---|---|---|---|
| Orlistat | 1.24 | −6.66 |
Asn385 | 2.85 2.26 |
Lys419 | - | |||
| Compound 1 | 1.78 | −7.70 |
Glu350 Thr355 Asp409 Asn295
| 1.96 1.83 2.13 2.79 2.61 - |
| Compound 2 | 1.99 | −7.20 |
Ser351 Asp409 Thr355 | 2.88 2.02 2.88 2.20 2.49 |
| Compound 3 | 1.60 | −7.39 |
Thr355 Asp409 Glu350 Asn353 Asn295 Asn411 Asp297
| 3.10 2.38 2.07 2.06 2.42 2.14 2.38 2.87 - |
| Compound 4 | 2.16 | −5.43 |
Gln384 Thr355 Asp409 Ser351 Glu350 | 2.34 2.88 2.08 2.09 2.15 2.98 2.03 |
| Compound 5 | 2.03 | −5.51 | - | 3.02 2.01 1.92 2.5 |
| α-Amylase Inhibition at 300 µg/mL | |||
| Tested Sample | % Inhibition | IC50 µg/mL | p Value |
| AEEE | 89.02 ± 2.31 | 176.3 ± 4.21 | |
| MGGG | 91.36 ± 2.45 | 157.54 ± 5.9 | <0.0001 |
| Kaempferol 3-O-rutinoside | 80.37 ± 3.81 * | 206.89 ± 5.60 | <0.0001 |
| Quercetin 3-O-rutinoside | 72.13 ± 3.49 | 223.6 ± 3.90 | <0.0001 |
| Tellimagranidine-I | 78.92 ± 4.13 | 217.35 ± 5.30 | <0.0001 |
| 2,3-α, β-digalloy glucose | 78.04 ± 1.15 | 216.89 ± 5.50 | <0.0001 |
| Acarbose 300 ug/mL | 93 ± 1.80 | 71.85 ± 3.70 | <0.0001 |
| β-glucosidase inhibition at 300 µg/mL | |||
| AEEE | 88.13 ± 1.25 | 76.12 ± 2.25 | <0.0001 |
| MGGG | 92.70 ± 1.42 * | 72.77 ± 2.67 | <0.0001 |
| Kaempferol 3-O-rutinoside | 47 ± 4.79 * | 140.33 ± 5.20 | <0.0001 |
| Quercetin 3-O-rutinoside | 66 ± 3.47 * | 104 ± 3.80 | <0.0001 |
| Tellimagranidine-I | 58.44 ± 3.61 * | 115.21± 4.90 | <0.0001 |
| 2,3-α, β-digalloy glucose | 68.43 ± 2.30 * | 100.23 ± 3.80 | <0.0001 |
| Acarbose at 600 µg/mL | 95.2 ± 2.30 | 110.6 ± 3.20 | <0.0001 |
| Pancreatic Lipase Inhibition at 100 µg/mL | |||
| AEEE | 79 ±3.21 | 45 ±5.11 | |
| MGGG | 87 ± 2.38 * | 32 ± 4.43 * | <0.0001 |
| Kaempferol 3-O-rutinoside | 35.81 ± 3.84 * | 135.8 ± 4.30 * | <0.0001 |
| Quercetin 3-O-rutinoside | 82.17 ± 2.53 * | 40.42 ± 2.41 * | <0.0001 |
| Tellimagranidine-I | 19.52 ± 4.36 * | 270.1 ± 6.20 * | <0.0001 |
| 2,3-α,β-digalloy glucose | 18.69 ± 3.10 * | 265.95± 4.71 * | <0.0001 |
| Orlistat 100 ug/mL | 57 ± 4.50 | 3.22 ± 1.30 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swilam, N.; Nawwar, M.A.M.; Radwan, R.A.; Mostafa, E.S. Antidiabetic Activity and In Silico Molecular Docking of Polyphenols from Ammannia baccifera L. subsp. Aegyptiaca (Willd.) Koehne Waste: Structure Elucidation of Undescribed Acylated Flavonol Diglucoside. Plants 2022, 11, 452. https://doi.org/10.3390/plants11030452
Swilam N, Nawwar MAM, Radwan RA, Mostafa ES. Antidiabetic Activity and In Silico Molecular Docking of Polyphenols from Ammannia baccifera L. subsp. Aegyptiaca (Willd.) Koehne Waste: Structure Elucidation of Undescribed Acylated Flavonol Diglucoside. Plants. 2022; 11(3):452. https://doi.org/10.3390/plants11030452
Chicago/Turabian StyleSwilam, Noha, Mahmoud A. M. Nawwar, Rasha A. Radwan, and Eman S. Mostafa. 2022. "Antidiabetic Activity and In Silico Molecular Docking of Polyphenols from Ammannia baccifera L. subsp. Aegyptiaca (Willd.) Koehne Waste: Structure Elucidation of Undescribed Acylated Flavonol Diglucoside" Plants 11, no. 3: 452. https://doi.org/10.3390/plants11030452