Monoterpene Synthase Genes and Monoterpene Profiles in Pinus nigra subsp. laricio
Abstract
:1. Introduction
1.1. Terpenoids: Basic Definitions and Roles in Conifers
1.2. Ecophysiology and Biotechnology of Conifer Monoterpenes
1.3. Biosynthesis of Monoterpenes in the Pinaceae: Genes and Enzymes
1.4. Aims and Background of the Present Work
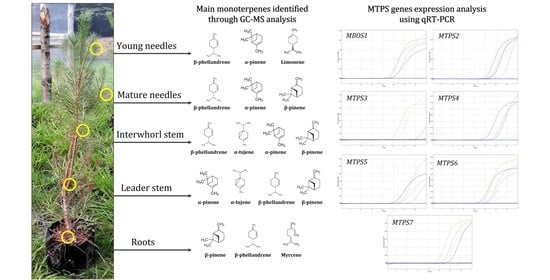
2. Results and Discussion
2.1. The Tissue-Specific and Species-Specific Monoterpene Metabolites in the Pinaceae
2.2. A Phylogeny-Based Approach for Isolating Partial and Full-Length cDNAs Coding for Monoterpene Synthases in P. laricio
2.3. Sequence-Based Analysis of the Predicted MBOS and MTPS Proteins in P. laricio
2.4. Genomic Organization of MBO/Monoterpene Synthases in P. laricio on the Background of MTPS Functional Evolution
2.5. Transcript Profiling of P. laricio Monoterpene Synthase Genes Reveals Differential Expression across Different Tissues and Suggests Their Putative Roles in the Biosynthesis of Monoterpenes
3. Materials and Methods
3.1. Plant Material
3.2. Extraction and GC–MS Analysis of Monoterpene Metabolites
3.3. Isolation, Characterization, and Expression Analyses of Monoterpene Synthases Genes in P. laricio
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Springer: Cham, Switzerland, 2015; pp. 63–106. [Google Scholar]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef]
- Tholl, D.; Lee, S. Terpene specialized metabolism in Arabidopsis thaliana. Arab. Book 2011, 9, e0143. [Google Scholar] [CrossRef] [Green Version]
- Celedon, J.M.; Bohlmann, J. Oleoresin defenses in conifers: Chemical diversity, terpene synthases and limitations of oleoresin defense under climate change. New Phytol. 2019, 224, 1444–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alicandri, E.; Paolacci, A.R.; Osadolor, S.; Sorgonà, A.; Badiani, M.; Ciaffi, M. On the evolution and functional diversity of terpene synthases in the Pinus species: A Review. J. Mol. Evol. 2020, 88, 253–283. [Google Scholar] [CrossRef]
- Zulak, K.G.; Bohlmann, J. Terpenoid biosynthesis and specialized vascular cells of conifer defense. J. Integr. Plant Biol. 2010, 52, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Raffa, K.F. Terpenes tell different tales at different scales: Glimpses into the chemical ecology of conifer-bark beetle-microbial interactions. J. Chem. Ecol. 2014, 40, 1–20. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Kopaczyk, J.M.; Warguła, J.; Jelonek, T. The variability of terpenes in conifers under developmental and environmental stimuli. Environ. Exp. Bot. 2020, 104197. [Google Scholar] [CrossRef]
- Nicole, M.C.; Zeneli, G.; Lavallée, R.; Rioux, D.; Bauce, É.; Morency, M.J.; Fenning, T.M.; Séguin, A. White pine weevil (Pissodes strobi) biological performance in un-affected by the jasmonic acid or wound-induced defense response in Norway spruce (Picea abies). Tree Physiol. 2006, 26, 1377–1389. [Google Scholar] [CrossRef]
- Hall, D.E.; Robert, J.A.; Keeling, C.I.; Domanski, D.; Quesada, A.L.; Jancsik, S.; Kuzyk, M.A.; Hamberger, B.; Borchers, C.H.; Bohlmann, J. An integrated genomic, proteomic and biochemical analysis of (+)-3-carene biosynthesis in Sitka spruce (Picea sitchensis) genotypes that are resistant or susceptible to white pine weevil: (+)-3-carene biosynthesis in Sitka spruce. Plant J. 2011, 65, 936–948. [Google Scholar] [CrossRef]
- Pollastrini, M.; Luchi, N.; Michelozzi, M.; Gerosa, G.; Marzuoli, R.; Bussotti, F.; Capretti, P. Early physiological responses of Pinus pinea L. seedlings infected by Heterobasidion sp. pl. in an ozone-enriched atmospheric environment. Tree Physiol. 2015, 35, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.E.; Yuen, M.M.; Jancsik, S.; Quesada, A.L.; Dullat, H.K.; Li, M.; Henderson, H.; Arango-Velez, A.; Liao, N.Y.; Docking, R.T.; et al. Transcriptome resources and functional characterization of monoterpene synthases for two host species of the mountain pine beetle, lodgepole pine (Pinus contorta) and jack pine (Pinus banksiana). BMC Plant Biol. 2013, 13, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boone, C.K.; Aukema, B.H.; Bohlmann, J.; Carroll, A.L.; Raffa, K.F. Efficacy of tree defense physiology varies with bark beetle population density: A basis for positive feedback in eruptive species. Can. J. For. Res. 2011, 41, 1174–1188. [Google Scholar] [CrossRef]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Gray, D.W.; Pell, H.K.; Breneman, S.R.; Topper, L. Isoprene synthase genes form a monophyletic clade of acyclic terpene synthases in the Tps-b terpene synthase family. Evolution 2013, 67, 1026–1040. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. In vitro neuroprotective potential of the monoterpenes α-pinene and 1, 8-cineole against H2O2-induced oxidative stress in PC12 cells. Z. Nat. C 2016, 71, 191–199. [Google Scholar] [CrossRef]
- George, K.W.; Alonso-Gutierrez, J.; Keasling, J.D.; Lee, T.S. Isoprenoid drugs, biofuels, and chemicals—Artemisinin, farnesene, and beyond. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Springer: Cham, Switzerland, 2015; pp. 355–390. [Google Scholar]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef]
- Sobral, M.V.; Xavier, A.L.; Lima, T.C.; de Sousa, D.P. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014, 2014, 953451. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.L.; Keeling, C.I.; Yuen, M.M.; Raymond, A.; Taylor, G.A.; Vandervalk, B.P.; Mohamadi, H.; Paulino, D.; Chiu, R.; Jackman, S.D.; et al. Improved white spruce (Picea glauca) genome assemblies and annotation of large gene families of conifer terpenoid and phenolic defense metabolism. Plant J. 2015, 83, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Keeling, C.I.; Weisshaar, S.; Ralph, S.G.; Jancsik, S.; Hamberger, B.; Dullat, H.K.; Bohlmann, J. Transcriptome mining, functional characterization, and phylogeny of a large terpene synthase gene family in spruce (Picea Spp.). BMC Plant Biol. 2011, 11, 43. [Google Scholar] [CrossRef] [Green Version]
- Foti, V.; Araniti, F.; Manti, F.; Alicandri, E.; Giuffrè, A.M.; Bonsignore, C.P.; Castiglione, E.; Sorgonà, A.; Covino, S.; Paolacci, A.R.; et al. Profiling volatile terpenoids from P. laricio stands infested by the pine processionary moth. Plants 2020, 9, 1362. [Google Scholar] [CrossRef]
- Alicandri, E.; Covino, S.; Sebastiani, B.; Paolacci, A.R.; Badiani, M.; Manti, F.; Bonsignore, C.P.; Sorgonà, A.; Ciaffi, M. Diterpene resin acids and olefins in P. laricio (Pinus nigra subsp. laricio (Poiret) Maire) oleoresin: GC-MS profiling of major diterpenoids in different plant organs, molecular identification and expression analysis of diterpene synthase genes. Plants 2021, 10, 2391. [Google Scholar] [CrossRef]
- Nicolaci, A.; Travaglini, D.; Menguzzato, G.; Nocentini, S.; Veltri, A.; Iovino, F. Ecological and anthropogenic drivers of P. laricio (Pinus nigra J.F. Arn. ssp. laricio (Poiret) Maire) distribution in the sila mountain range. iForest 2015, 8, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Geron, C.; Rasmussen, R.; Arnts, R.R.; Guenther, A. A review and synthesis of monoterpene speciation from forests in the United States. Atmos. Environ. 2000, 34, 1761–1781. [Google Scholar] [CrossRef] [Green Version]
- Pokorska, O.; Dewulf, J.; Amelynck, C.; Schoon, N.; Šimpraga, M.; Steppe, K.; Van Langenhove, H. Isoprene and terpenoid emissions from Abies alba: Identification and emission rates under ambient conditions. Atmos. Environ. 2012, 59, 501–508. [Google Scholar] [CrossRef]
- Blanch, J.S.; Penuelas, J.; Sardans, J.; Llusia, J. Drought, warming and soil fertilization effects on leaf volatile terpene concentrations in Pinus halepensis and Quercus ilex. Acta Physiol. Plant 2009, 31, 207–218. [Google Scholar] [CrossRef]
- Mukrimin, M.; Kovalchuk, A.; Ghimire, R.P.; Kivimäenpää, M.; Sun, H.; Holopainen, J.K.; Asiegbu, F.O. Evaluation of potential genetic and chemical markers for Scots pine tolerance against Heterobasidion annosum infection. Planta 2019, 250, 1881–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, D.W.; Breneman, S.R.; Topper, L.A.; Sharkey, T.D. Biochemical characterization and homology modeling of methylbutenol synthase and implications for understanding hemiterpene synthase evolution in plants. J. Biol. Chem. 2011, 286, 20582–20590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittington, D.A.; Wise, M.L.; Urbansky, M.; Coates, R.M.; Croteau, R.B.; Christianson, D.W. Bornyl diphosphate synthase: Structure and strategy for carbocation manipulation by a terpenoid cyclase. Proc. Natl. Acad. Sci. USA 2002, 99, 15375–15380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyatt, D.C.; Youn, B.; Zhao, Y.; Santhamma, B.; Coates, R.M.; Croteau, R.B.; Kang, C. Structure of limonene synthase, a simple model for terpenoid cyclase catalysis. Proc. Natl. Acad. Sci. USA 2007, 104, 5360–5365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarshis, L.C.; Proteau, P.J.; Kellogg, B.A.; Sacchettini, J.C.; Poulter, C.D. Regulation of product chain length by isoprenyl diphosphate synthases. Proc. Natl. Acad. Sci. USA 1996, 93, 15018–15023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesburg, C.A.; Zhai, G.; Cane, D.E.; Christianson, D.W. Crystal structure of pentalenene synthase: Mechanistic insights on terpenoid cyclization reactions in biology. Science 1997, 277, 1820–1824. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.A.; Wildung, M.R.; Williams, D.C.; Hyatt, D.C.; Croteau, R. cDNA isolation, functional expression, and characterization of (+)-α-pinene synthase and (−)-α-pinene synthase from loblolly pine (Pinus taeda): Stereocontrol in pinene biosynthesis. Arch. Biochem. Biophys. 2003, 411, 267–276. [Google Scholar] [CrossRef]
- Lehnert, A.S.; Perreca, E.; Gershenzon, J.; Pohnert, G.; Trumbore, S.E. Simultaneous real-time measurement of isoprene and 2-methyl-3-buten-2-ol emissions from trees using SIFT-MS. Front. Plant Sci. 2020, 11, 1867. [Google Scholar] [CrossRef]
- Trapp, S.C.; Croteau, R.B. Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 2001, 158, 811–832. [Google Scholar] [CrossRef]
- Hamberger, B.; Hall, D.; Yuen, M.; Oddy, C.; Hamberger, B.; Keeling, C.I.; Ritland, C.; Ritland, K.; Bohlmann, J. Targeted isolation, sequence assembly and characterization of two white spruce (Picea glauca) BAC clones for terpenoid synthase and cytochrome P450 genes involved in conifer defence reveal insights into a conifer genome. BMC Plant Biol. 2009, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.W.S.; Simpson, C.G. Splice site selection in plant pre-mRNA splicing. Annu. Rev. Plant Biol. 1998, 49, 77–95. [Google Scholar] [CrossRef]
- Li, W.-H. Molecular Evolution; Sinauer Associates: Sunderland, MA, USA, 1997. [Google Scholar]
- Lerdau, M.; Gray, D. Ecology and evolution of light-dependent and light-independent phytogenic volatile organic carbon. New Phytol. 2003, 157, 199–211. [Google Scholar] [CrossRef]
- Loreto, F. Reconciling functions and evolution of isoprene emission in higher plants. New Phytol. 2015, 206, 578–582. [Google Scholar] [CrossRef]
- Harley, P.; Fridd-Stroud, V.; Greenberg, J.; Guenther, A.; Vasconcellos, P. Emission of 2-methyl-3-buten-2-ol by pines: A potentially large natural source of reactive carbon to the atmosphere. J. Geophys. Res. 1998, 103, 25479–25486. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.M.; Fäldt, J.; Bohlmann, J. Functional characterization of nine Norway spruce TPS genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily. Plant Physiol. 2004, 135, 1908–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun-McKay, A.; Godard, K.A.; Toudefallah, M.; Martin, D.M.; Alfaro, R.; King, J.; Bohlmann, J.; Plant, A.L. Wound-induced terpene synthase gene expression in Sitka spruce that exhibit resistance or susceptibility to attack by the white pine weevil. Plant Physiol. 2006, 140, 1009–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohlmann, J.; Steele, C.L.; Croteau, R. Monoterpene synthases from grand fir (Abies grandis): cDNA isolation, characterization, and functional expression of myrcene synthase, (−)-(4S)-limonene synthase, and (−)-(1S, 5S)-pinene synthase. J. Biol. Chem. 1997, 272, 21784–21792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wise, M.L.; Savage, T.J.; Katahira, E.; Croteau, R. Monoterpene synthases from common sage (Salvia officinalis): cDNA isolation, characterization, and functional expression of (+)-sabinene synthase, 1, 8-cineole synthase, and (+)-bornyl diphosphate synthase. J. Biol. Chem. 1998, 273, 14891–14899. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.T.; Chu, F.H. Molecular cloning and characterization of monoterpene synthases from Litsea cubeba (Lour.) Persoon. Tree Genet. 2011, 7, 835–844. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Ciaffi, M.; Paolacci, A.R.; Paolocci, M.; Alicandri, E.; Bigini, V.; Badiani, M.; Muganu, M. Transcriptional regulation of stilbene synthases in grapevine germplasm differentially susceptible to downy mildew. BMC Plant Biol. 2019, 19, 1–18. [Google Scholar] [CrossRef]
- Paolacci, A.R.; Catarcione, G.; Ederli, L.; Zadra, C.; Pasqualini, S.; Badiani, M.; Musetti, R.; Santi, S.; Ciaffi, M. Jasmonate-mediated defence responses, unlike salicylate-mediated responses, are involved in the recovery of grapevine from bois noir disease. BMC Plant Biol. 2017, 17, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Intron | MBOS1 | MTPS2 | MTPS3 | MTPS4 | MTPS5 | MTPS6 | MTPS7 |
|---|---|---|---|---|---|---|---|
| I | - | - | - | - | - | - | - |
| II | - | - | - | - | - | - | - |
| III | G74 (97) | E87 (318) | G86 (177) | Y91 (102) | G89 (123) | E87 (91) | G88 (183) |
| IV | - | - | - | - | - | - | - |
| V | - | - | - | - | - | - | - |
| VI | - | - | - | - | - | - | - |
| VII | Y141 ** (159) | S153 ** (79) | R154 ** (81) | S156 ** (124) | S157 ** (90) | S155 ** (90) | S156 ** (89) |
| VIII | S179 * (115) | S190 * (101) | S191 * (106) | A193 * (148) | A195 * (80) | S192 * (111) | S193 * (104) |
| IX | E249 (94) | Q261 (225) | E261 (124) | E263 (263) | E271 (74) | E262 (97) | E263 (86) |
| X | L281 ** (269) | E290 ** (289) | S292 ** (186) | T292 ** (69) | E301 ** (181) | Q295 ** (221) | I293 ** (297) |
| XI | R314 ** (105) | R326 ** (108) | R326 ** (91) | R328 ** (125) | R336 ** (93) | R321 ** (94) | R328 ** (98) |
| XII | R386 ** (111) | R398 ** (111) | R398 ** (114) | R400 ** (91) | R408 ** (92) | R393 ** (98) | R400 ** (208) |
| XIII | A432 (99) | A444 (89) | A444 (100) | A446 (108) | A454 (111) | W440 (113) | A446 (89) |
| XIV | Q515 (94) | Q527 (112) | G543 (133) | Q529 (469) | Q537 (99) | A523 (112) | K529 (88) |
| Genomic size (bp) | 2988 | 3313 | 2978 | 3386 | 2854 | 2893 | 3132 |
| Protein length (aa) | 614 | 626 | 621 | 628 | 636 | 621 | 629 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alicandri, E.; Covino, S.; Sebastiani, B.; Paolacci, A.R.; Badiani, M.; Sorgonà, A.; Ciaffi, M. Monoterpene Synthase Genes and Monoterpene Profiles in Pinus nigra subsp. laricio. Plants 2022, 11, 449. https://doi.org/10.3390/plants11030449
Alicandri E, Covino S, Sebastiani B, Paolacci AR, Badiani M, Sorgonà A, Ciaffi M. Monoterpene Synthase Genes and Monoterpene Profiles in Pinus nigra subsp. laricio. Plants. 2022; 11(3):449. https://doi.org/10.3390/plants11030449
Chicago/Turabian StyleAlicandri, Enrica, Stefano Covino, Bartolomeo Sebastiani, Anna Rita Paolacci, Maurizio Badiani, Agostino Sorgonà, and Mario Ciaffi. 2022. "Monoterpene Synthase Genes and Monoterpene Profiles in Pinus nigra subsp. laricio" Plants 11, no. 3: 449. https://doi.org/10.3390/plants11030449