Biogenic CuO and ZnO Nanoparticles as Nanofertilizers for Sustainable Growth of Amaranthus hybridus
Abstract
:1. Introduction
2. Materials and Methods
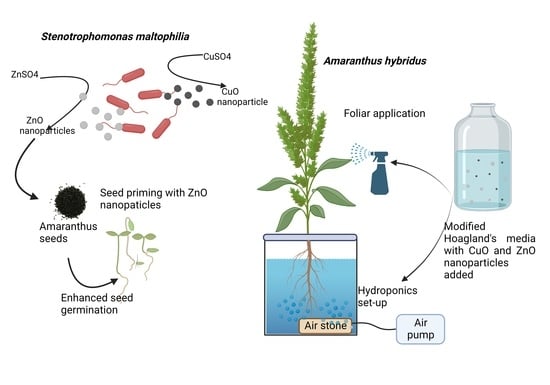
2.1. Synthesis of CuO and ZnO Nanoparticle
2.2. Seed Priming
2.3. Composition of Modified Hoagland’s Medium [35]
2.4. Selecting the Optimal Concentration of CuO and ZnO Nanoparticles for Plant Growth
2.5. Growth in Hydroponic System and Pot Study
2.5.1. Foliar Application
2.5.2. Hydroponic System
2.6. Chlorophyll Measurement Using SPAD
2.7. Preparation of Leaf Extract
2.8. Estimation of Total Reducing Sugar
2.9. Antioxidant Activity Assay
2.10. Total Phenolic Contents
2.11. Total Flavonoid Contents
2.12. Estimation of Cu2+ and Zn2+ Ions
2.13. Statistical Analysis
3. Results
3.1. Characterization of Biogenic CuO and ZnO Nanoparticles
3.2. Seed Priming
3.3. Optimal Concentration of CuO and ZnO Nanoparticles
3.4. Growth of the Plants in Pot Study and Hydroponic System
3.4.1. Foliar Application of Fertilizers
3.4.2. Hydroponics Application of Fertilizer
3.5. Chlorophyll Measurement in Plants Treated with CuO and ZnO Nanoparticles
3.6. Estimation of Reducing Sugar in Plants Treated with CuO and ZnO Nanoparticles
3.7. Antioxidant Activity
3.8. Total Phenolic Content
3.9. Total Flavonoid Content
3.10. Uptake of Copper and Zinc in Plant Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duro, J.A.; Lauk, C.; Kastner, T.; Erb, K.H.; Haberl, H. Global inequalities in food consumption, cropland demand and land-use efficiency: A decomposition analysis. Glob. Environ. Change 2020, 64, 102124. [Google Scholar] [CrossRef]
- FAO Statistical Yearbook, 2021-World Food and Agriculture. Available online: https://reliefweb.int/report/world/fao-statistical-yearbook-2021-world-food-and-agriculture (accessed on 19 August 2022).
- Ray, D.K.; Ramankutty, N.; Mueller, N.D.; West, P.C.; Foley, J.A. Recent patterns of crop yield growth and stagnation. Nat. Commun. 2012, 3, 1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horton, P.; Long, S.P.; Smith, P.; Banwart, S.A.; Beerling, D.J. Technologies to deliver food and climate security through agriculture. Nat. Plants 2021, 7, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Maltas, A.; Kebli, H.; Oberholzer, H.R.; Weisskopf, P.; Sinaj, S. The effects of organic and mineral fertilizers on carbon sequestration, soil properties, and crop yields from a long-term field experiment under a Swiss conventional farming system. Land Degrad. Dev. 2018, 29, 926–938. [Google Scholar] [CrossRef]
- Bhatt, M.K.; Labanya, R.; Joshi, H.C. Influence of long-term chemical fertilizers and organic manures on soil fertility—A review. Univers. J. Agric. Res. 2019, 7, 177–188. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y.; Saifullah; Ahmad, M. Improving agricultural water use efficiency by nutrient management in crop plants. Acta Agric. Scand. Sect. B Soil Plant Sci. 2011, 61, 291–304. [Google Scholar] [CrossRef]
- Powlson, D.S.; Gregory, P.J.; Whalley, W.R.; Quinton, J.N.; Hopkins, D.W.; Whitmore, A.P.; Hirsch, P.R.; Goulding, K.W. Soil management in relation to sustainable agriculture and ecosystem services. Food Policy 2011, 36, S72–S87. [Google Scholar] [CrossRef]
- Francis, D.V.; Sood, N.; Gokhale, T. Progress and Prospects in Nanoscience Today; Shivaji Pawar, Ed.; Nova Science Publishers: New York, NY, USA, 2020; pp. 157–178. ISBN 978-1-53617-292-8. [Google Scholar]
- Muraisi, L.; Hariyadi, D.M.; Athiyah, U.; Pathak, Y. Eco-friendly Nanotechnology in Agriculture: Opportunities, Toxicological Implications, and Occupational Risks. In Sustainable Nanotechnology: Strategies, Products, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 287–296. [Google Scholar] [CrossRef]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Ilyas, A.; Ashraf, M.Y.; Hussain, M.; Ashraf, M.; Ahmed, R.; Kamal, A. Effect of micronutrients (Zn, Cu and B) on photosynthetic and fruit yield attributes of citrus reticulata Blanco var. kinnow. Pak. J. Bot. 2015, 47, 1241–1247. [Google Scholar]
- Ducic, T.; Polle, A. Transport and detoxification of manganese and copper in plants. Braz. J. Plant Physiol. 2005, 17, 103–112. [Google Scholar] [CrossRef]
- Ravet, K.; Pilon, M. Copper and iron homeostasis in plants: The challenges of oxidative stress. Antioxid. Redox Signal. 2013, 19, 919–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayu, T. Review on contribution of integrated soil fertility management for climate change mitigation and agricultural sustainability. Cogent Environ. Sci. 2020, 6, 1823631. [Google Scholar] [CrossRef]
- Weng, S.; Hu, X.; Wang, J.; Tang, L.; Li, P.; Zheng, S.; Zheng, L.; Huang, L.; Xin, Z. Advanced application of Raman spectroscopy and surface-enhanced Raman spectroscopy in plant disease diagnostics: A review. J. Agric. Food Chem. 2021, 69, 2950–2964. [Google Scholar] [CrossRef] [PubMed]
- Özkara, A.; Akyıl, D.; Konuk, M. Pesticides, environmental pollution, and health. In Environmental Health Risk-Hazardous Factors to Living Species; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Fatima, F.; Hashim, A.; Anees, S. Efficacy of nanoparticles as nanofertilizer production: A review. Environ. Sci. Pollut. Res. 2021, 28, 1292–1303. [Google Scholar] [CrossRef]
- Pirzadah, B.; Pirzadah, T.B.; Jan, A.; Hakeem, K.R. Nanofertilizers: A way forward for green economy. In Nanobiotechnology in Agriculture; Springer: Cham, Switzerland, 2020; pp. 99–112. [Google Scholar] [CrossRef]
- Manjunatha, S.B.; Biradar, D.P.; Aladakatti, Y.R. Nanotechnology and its applications in agriculture: A review. J. Farm. Sci. 2016, 29, 1–3. [Google Scholar]
- Iqbal, M.A. Nano-fertilizers for sustainable crop production under changing climate: A global perspective. Sustain. Crop. Prod. 2019, 8, 89089. [Google Scholar]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Midmore, D.; Deng-Lin, W. Work that water! Hydroponics made easy. Waterlines 1999, 17, 28–30. [Google Scholar] [CrossRef]
- Rios, J.J.; Yepes-Molina, L.; Martinez-Alonso, A.; Carvajal, M. Nanobiofertilization as a novel technology for highly efficient foliar application of Fe and B in almond trees. R. Soc. Open Sci. 2020, 7, 200905. [Google Scholar] [CrossRef]
- García-López, J.I.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.H.; Díaz Barriga-Castro, E.; Ruiz-Torres, N.A.; Niño-Medina, G. Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum annuum L. during germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Abbasifar, A.; Shahrabadi, F.; ValizadehKaji, B. Effects of green synthesized zinc and copper nano-fertilizers on the morphological and biochemical attributes of basil plant. J. Plant Nutr. 2020, 43, 1104–1118. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Kumar, A. Impact of irrigation using water containing CuO and ZnO nanoparticles on Spinach oleracea grown in soil media. Bull. Environ. Contam. Toxicol. 2016, 97, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.W.; Wu, T.H.; Wang, S.T. Vegetable amaranth (Amaranthus L.); AVRDC Publication: Tainan, Taiwan, 2011; Volume 9, pp. 11–754. [Google Scholar]
- Francis, D.V.; Thaliyakattil, S.; Cherian, L.; Sood, N.; Gokhale, T. Metallic Nanoparticle Integrated Ternary Polymer Blend of PVA/Starch/Glycerol: A Promising Antimicrobial Food Packaging Material. Polymers 2022, 14, 1379. [Google Scholar] [CrossRef]
- Handayani, E.; Irsyadi, M.B.; Alawiyah RL, M.N.; Aris, I. Effect of Explants Sterilization and Plant Growth Regulators on Embryo Culture of Kepel (Stelechocarpus burahol). IOP Conf. Ser. Earth Environ. Sci. 2022, 985, 012016. [Google Scholar] [CrossRef]
- Imran, M.; Mahmood, A.; Neumann, G.; Boelt, B. Zinc Seed Priming Improves Spinach Germination at Low Temperature. Agriculture 2021, 11, 271. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Zhu, J.; Tremblay, N.; Liang, Y. Comparing SPAD and at LEAF values for chlorophyll assessment in crop species. Can. J. Soil Sci. 2012, 92, 645–648. [Google Scholar] [CrossRef]
- Ludwig, T.G.; Goldberg, H.J.V. The Anthrone Method for the Determination of carbohydrates in Foods and in Oral Rinsing. J. Dent. Res. 1956, 35, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Arthur Thomas, T. An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agric. 1977, 28, 639–642. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 1, 669–675. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Antioxidant and phytochemical activities of Amaranthus caudatus L. harvested from different soils at various growth stages. Sci. Rep. 2019, 9, 12965. [Google Scholar] [CrossRef] [Green Version]
- Carnat, A.; Carnat, A.P.; Fraisse, D.; Lamaison, J.L. The aromatic and polyphenolic composition of lemon verbena tea. Fitoterapia. 1999, 70, 44–49. [Google Scholar] [CrossRef]
- Santos, A.D.; Matos, R.A.; Andrade, E.M.; dos Santos, W.N.; Magalhães, H.I.; Costa, F.D.; Korn, M.D. Multielement determination of macro and micro contents in medicinal plants and phytomedicines from Brazil by ICP OES. J. Braz. Chem. Soc. 2017, 28, 376–384. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, S.F. Analysis of variance: The fundamental concepts. J. Man. Manip. Ther. 2009, 17, 27E–38E. [Google Scholar] [CrossRef]
- Gerald, B. A brief review of independent, dependent and one sample t-test. Int. J. Appl. Math. Theor. Phys. 2018, 14, 50–54. [Google Scholar] [CrossRef]
- Bacha, R.; Bouchtout, A.L.; Boulares, N.; Mahcene, F.; Chari, A.; Chaieb, A. Synthesis and Characterization of CuO Nano Particles. In Proceedings of the 21st International Conference on Transparent Optical Networks (ICTON), Angers, France, 9–13 July 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Yang, W.; Zhu, Z.; Wu, Y. Positron annihilation spectroscopy study on annealing effect of CuO nanoparticles. Mater. Res. 2016, 19, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Ebin, B.; Arıg, E.; Özkal, B.; Gürmen, S. Production and characterization of ZnO nanoparticles and porous particles by ultrasonic spray pyrolysis using a zinc nitrate precursor. Int. J. Miner. Metall. Mater. 2012, 19, 651–656. [Google Scholar] [CrossRef]
- Bodade, A.B.; Taiwade, M.A.; Chaudhari, G.N. Bioelectrode based chitosan-nano copper oxide for application to lipase biosensor. J. Appl. Pharm. Res. 2017, 5, 30–39. [Google Scholar]
- Miller, L.M.; Bourassa, M.W.; Smith, R.J. FTIR spectroscopic imaging of protein aggregation in living cells. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2339–2346. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Cui, J.; Zhang, H.; Wang, G.; Zhao, F.J.; Shen, Z. Proteomic analysis of copper stress responses in the roots of two rice (Oryza sativa L.) varieties differing in Cu tolerance. Plant Soil 2013, 366, 647–658. [Google Scholar] [CrossRef]
- Arif, N.; Yadav, V.; Singh, S.; Singh, S.; Ahmad, P.; Mishra, R.K.; Sharma, S.; Tripathi, D.K.; Dubey, N.K.; Chauhan, D.K. Influence of high and low levels of plant beneficial heavy metal ions on plant growth and development. Front. Environ. Sci. Eng. 2016, 4, 69. [Google Scholar] [CrossRef]
- Xin, X.; Zhao, F.; Rho, J.Y.; Goodrich, S.L.; Sumerlin, B.S.; He, Z. Use of polymeric nanoparticles to improve seed germination and plant growth under copper stress. Sci. Total Environ. 2020, 745, 141055. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Lu, Y.; Liao, Y.; Nie, J.; Yuan, X.; Chen, F. Use of a leaf chlorophyll content index to improve the prediction of above-ground biomass and productivity. PeerJ 2019, 6, e6240. [Google Scholar] [CrossRef] [Green Version]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Wang, R.; Wang, R.; Zhang, P.; Ju, Q.; Xu, J. Physiological, transcriptomic, and metabolomic analyses reveal zinc oxide nanoparticles modulate plant growth in tomato. Environ. Sci. Nano 2020, 7, 3587–3604. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Alzahrani, H.S. The potential mitigation effect of ZnO nanoparticles on [Abelmoschus esculentus L. Moench] metabolism under salt stress conditions. Saudi J. Biol. Sci. 2020, 27, 3132–3137. [Google Scholar] [CrossRef] [PubMed]
- Droppa, M.; Horváth, G. The role of copper in photosynthesis. Crit. Rev. Plant Sci. 1990, 9, 111–123. [Google Scholar] [CrossRef]
- Shakya, K.; Chettri, M.K.; Sawidis, T. Impact of heavy metals (copper, zinc, and lead) on the chlorophyll content of some mosses. Arch. Environ. Contam. Toxicol. 2008, 54, 412–421. [Google Scholar] [CrossRef]
- Azizollahi, Z.; Ghaderian, S.M.; Ghotbi-Ravandi, A.A. Cadmium accumulation and its effects on physiological and biochemical characters of summer savory (Satureja hortensis L.). Int. J. Phytoremediation 2019, 21, 1241–1253. [Google Scholar] [CrossRef]
- Bang, J.H.; Lee, K.J.; Jeong, W.T.; Han, S.; Jo, I.-H.; Choi, S.H.; Cho, H.; Hyun, T.K.; Sung, J.; Lee, J.; et al. Antioxidant Activity and Phytochemical Content of Nine Amaranthus Species. Agronomy 2021, 11, 1032. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; ur Rehman, M.Z.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2022, 423, 127021. [Google Scholar] [CrossRef]
- Arun, M.N.; Hebbar, S.S.; Senthivel, T.; Nair, A.K.; Padmavathi, G.; Pandey, P.; Singh, A. Seed Priming: The Way Forward to Mitigate Abiotic Stress in Crops. In Plant Stress Physiology-Perspectives in Agriculture; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Dembélé, S. Developing Cultivation Practices to Combat Early Drought Challenges: The case of Sorghum in Mali. Ph.D. Thesis, University of Cape Coast, Cape Coast, Ghana, 2016. [Google Scholar]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Ahmad, R.; Basra SM, A. Seed priming with zinc improves the germination and early seedling growth of wheat. Seed Sci. Technol. 2015, 43, 262–268. [Google Scholar] [CrossRef]
- Al-Mayahi, A.M.W. The effect of humic acid (HA) and zinc oxide nanoparticles (ZnO-NPS) on in vitro regeneration of date palm (Phoenix dactylifera L.) cv. Quntar. Plant Cell Tissue Organ Cult. 2021, 145, 445–456. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S.; Alok, A.; Upadhyay, S.K.; Rawat, M.; Tsang, D.C.; Bolan, N.; Kim, K.H. The potential of green synthesized zinc oxide nanoparticles as nutrient source for plant growth. J. Clean. Prod. 2019, 214, 1061–1070. [Google Scholar] [CrossRef]
- Mousavi, S.R. Zinc in crop production and interaction with phosphorus. Aust. J. Basic Appl. Sci. 2011, 5, 1503–1509. [Google Scholar]
- Coolong, T.W.; Randle, W.M. Zinc Concentration in Hydroponic Solution Culture Influences zinc and Sulfur Accumulation in Brassica rapa L. J. Plant Nutr. 2003, 26, 949–959. [Google Scholar] [CrossRef]
- Skiba, E.; Michlewska, S.; Pietrzak, M.; Wolf, W.M. Additive interactions of nanoparticulate ZnO with copper, manganese and iron in Pisum sativum L., a hydroponic study. Sci. Rep. 2020, 10, 13574. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, T.V.; Hebsur, N.S. Effect of nanofertilizers on growth and yield of selected cereals—A review. Agric. Rev. 2017, 38, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Babu, S.; Singh, R.; Yadav, D.; Rathore, S.S.; Raj, R.; Avasthe, R.; Yadav, S.K.; Das, A.; Yadav, V.; Yadav, B.; et al. Nanofertilizers for agricultural and environmental sustainability. Chemosphere 2022, 292, 133451. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. Biometals 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Shende, S.; Rathod, D.; Gade, A.; Rai, M. Biogenic copper nanoparticles promote the growth of pigeon pea (Cajanus cajan L.). IET Nanobiotechnol. 2016, 11, 773–781. [Google Scholar] [CrossRef]
- Martins, L.L.; Mourato, M.P. Effect of excess copper on tomato plants: Growth parameters, enzyme activities, chlorophyll, and mineral content. J. Plant Nutr. 2006, 29, 2179–2198. [Google Scholar] [CrossRef]
- Rani, N.; Kumari, K.; Sangwan, P.; Barala, P.; Yadav, J.; Hooda, V. Nano-iron and nano-zinc induced growth and metabolic changes in Vigna radiata. Sustainability 2022, 14, 8251. [Google Scholar] [CrossRef]
- Dammak, M.; Hadrich, B.; Miladi, R.; Barkallah, M.; Hentati, F.; Hachicha, R.; Laroche, C.; Michaud, P.; Fendri, I.; Abdelkafi, S. Effects of nutritional conditions on growth and biochemical composition of Tetraselmis sp. Lipids Health Dis. 2017, 16, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Yang, S.; Guo, R.; Guo, J. Warming and nitrogen addition alter photosynthetic pigments, sugars and nutrients in a temperate meadow ecosystem. PLoS ONE 2016, 11, e0155375. [Google Scholar] [CrossRef] [Green Version]
- Chalker-Scott, L.; Fuchigami, L.H. The role of phenolic compounds in plant stress responses. In Low Temperature Stress Physiology in Crops; CRC Press: Boca Raton, FL, USA, 2018; pp. 67–80. [Google Scholar]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad MN, V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef]
- Kachel, M.; Rudy, S.; Krajewska, M.; Rudy, M. Impact of Inorganic Metal (Ag, Cu) Nanoparticles on the Quality of Seeds and Dried Rapeseed Sprouts. Agriculture 2022, 12, 106. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Jacobo-Velázquez, D.A. Classification of phenolic compounds. In Phenolic Compounds in Food; CRC Press: Boca Raton, FL, USA, 2018; pp. 3–20. [Google Scholar]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Song, C.Z.; Liu, M.Y.; Meng, J.F.; Chi, M.; Xi, Z.M.; Zhang, Z.W. Promoting effect of foliage sprayed zinc sulfate on accumulation of sugar and phenolics in berries of Vitis vinifera cv. Merlot growing on zinc deficient soil. Molecules 2015, 20, 2536–2554. [Google Scholar] [CrossRef] [Green Version]
- Srivastav, A.; Ganjewala, D.; Singhal, R.K.; Rajput, V.D.; Minkina, T.; Voloshina, M.; Srivastava, S.; Shrivastava, M. Effect of ZnO Nanoparticles on Growth and Biochemical Responses of Wheat and Maize. Plants 2021, 10, 2556. [Google Scholar] [CrossRef] [PubMed]
- Waśkiewicz, A.; Muzolf-Panek, M.; Goliński, P. Phenolic content changes in plants under salt stress. In Ecophysiology and Responses of Plants under Salt Stress; Springer: New York, NY, USA, 2013; pp. 283–314. [Google Scholar] [CrossRef]
- Ferdinando, M.D.; Brunetti, C.; Fini, A.; Tattini, M. Flavonoids as antioxidants in plants under abiotic stresses. In Abiotic Stress Responses Plants; Springer Science+Business Media, LLC: New York, NY, USA, 2012; pp. 159–179. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-biotechnology in agriculture: Use of nanomaterials to promote plant growth and stress tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Tighe-Neira, R.; Gonzalez-Villagra, J.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Impact of nanoparticles and their ionic counterparts derived from heavy metals on the physiology of food crops. Plant Physiol. Biochem. 2022, 172, 14–23. [Google Scholar] [CrossRef]
- Copper- Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Copper-HealthProfessional/#:~:text=)%20%5B14%5D.-,Copper%20Intakes%20and%20Status,and%201%2C100%20mcg%20for%20women (accessed on 19 August 2022).
- Available online: https://ods.od.nih.gov/factsheets/Zinc-Consumer/ (accessed on 19 August 2022).
- Singh, D.; Kumar, A. Investigating long-term effect of nanoparticles on growth of Raphanus sativus plants: A trans-generational study. Ecotoxicology 2018, 27, 23–31. [Google Scholar] [CrossRef] [PubMed]











| Peak Position (2θ) | FWHM (θ) | Crystallite Size (nm) | Average Crystallite Size (nm) | |
|---|---|---|---|---|
| CuO | 20.90853 | 0.22918 | 35.24957 | 38.96 (±5) |
| 35.37912 | 0.27172 | 30.68807 | ||
| 38.61757 | 0.1803 | 46.68871 | ||
| 48.62369 | 0.27196 | 32.05383 | ||
| 61.41574 | 0.25925 | 35.64097 | ||
| 66.0172 | 0.17732 | 53.42576 | ||
| ZnO | 23.28048 | 0.26135 | 31.03541 | 42.64 (±3) |
| 31.8997 | 0.24101 | 34.28227 | ||
| 33.47986 | 0.21567 | 38.46548 | ||
| 34.76525 | 0.17377 | 47.90504 | ||
| 50.91306 | 0.14305 | 61.50663 |
| Foliar | Hydroponics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CuO 0.06 µm | CuO 0.12 µm | ZnO 0.12 µm | ZnO 0.24 µm | Water Control | Media Control | CuO 0.06 µm | CuO 0.12 µm | ZnO 0.12 µm | ZnO 0.24 µm | Water Control | Media Control | |
| No: of leaves | 26 | 28 | 27 | 32.3 | 15.5 | 13.5 | 31.3 | 41 | 42 | 38 | 15.3 | 14.3 |
| Leaf surface-area (cm2) | 62.5 | 59.2 | 69.2 | 67.5 | 39.7 | 38.4 | 63 | 67.8 | 72.7 | 78 | 51 | 36 |
| Total length (cm) | 50.4 | 51.7 | 44.4 | 62.1 | 40 | 36 | 56.8 | 58.2 | 58.7 | 63.1 | 41.7 | 40 |
| Total fresh weight (g) | 31 | 30.8 | 27.4 | 40.3 | 14 | 7.45 | 43.3 | 54 | 51.2 | 66.1 | 22 | 13.7 |
| Total dry weight (g) | 3.8 | 3.9 | 3.6 | 5.4 | 1.7 | 0.9 | 5.5 | 7.1 | 6.7 | 8.6 | 2.8 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francis, D.V.; Sood, N.; Gokhale, T. Biogenic CuO and ZnO Nanoparticles as Nanofertilizers for Sustainable Growth of Amaranthus hybridus. Plants 2022, 11, 2776. https://doi.org/10.3390/plants11202776
Francis DV, Sood N, Gokhale T. Biogenic CuO and ZnO Nanoparticles as Nanofertilizers for Sustainable Growth of Amaranthus hybridus. Plants. 2022; 11(20):2776. https://doi.org/10.3390/plants11202776
Chicago/Turabian StyleFrancis, Dali Vilma, Neeru Sood, and Trupti Gokhale. 2022. "Biogenic CuO and ZnO Nanoparticles as Nanofertilizers for Sustainable Growth of Amaranthus hybridus" Plants 11, no. 20: 2776. https://doi.org/10.3390/plants11202776