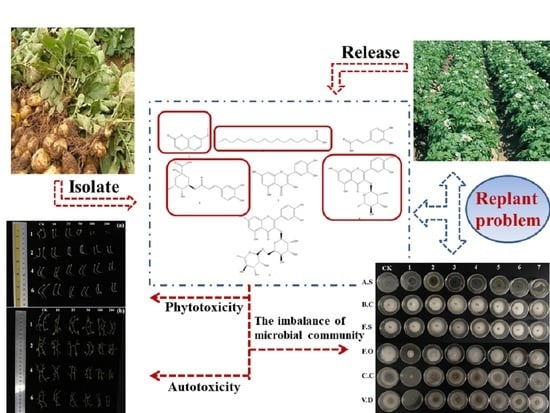
Allelochemicals from the Rhizosphere Soil of Potato (Solanum tuberosum L.) and Their Interactions with the Soilborne Pathogens
Abstract
:1. Introduction
2. Results
2.1. Allelopathic Activity of the Crude Extract of the Rhizosphere Soil of Potato
2.2. Isolation of Allelochemicals from the Rhizosphere Soil of Potato
2.3. Allelopathic Activities of the Purified Compounds on L. sativa Seedlings
2.4. Autotoxic Activities of the Purified Compounds on Tissue Culture Seedlings of Potato
2.5. Confirmation and Quantification of the Allelochemicals in the Rhizosphere Soil
2.6. Antifungal Activity on Potato Pathogens of the Allelochemicals
3. Discussion
4. Materials and Methods
4.1. General Experimental Instruments and Reagents
4.2. Soil Samples
4.3. Isolation of Allelochemicals from the Rhizosphere Soil
4.4. Plant Material and Growth Conditions
4.5. Bioassays
4.6. High-Performance Liquid Chromatography (HPLC) Analysis
4.7. Antifungal Activity
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lopez, A.; Arazuri, S.; Garcia, I.; Mangado, J.; Jaren, C. A review of the application of near-infrared spectroscopy for the analysis of potatoes. J. Agric. Food Chem. 2013, 61, 5413–5424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/about/en (accessed on 1 January 2022).
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Sakuma, R.; Ichisawa, M.; Ishihara, K.; Kubo, M.; Handa, S.; Mugita, H.; Maruyama, N.; Koga, H.; Ishigami, A. Potato chip intake increases ascorbic acid levels and decreases reactive oxygen species in SMP30/GNL knockout mouse tissues. J. Agric. Food Chem. 2014, 62, 9286–9295. [Google Scholar] [CrossRef] [PubMed]
- Orawetz, T.; Malinova, I.; Orzechowski, S.; Fettke, J. Reduction of the plastidial phosphorylase in potato (Solanum tuberosum L.) reveals impact on storage starch structure during growth at low temperature. Plant Physiol. Biochem. 2016, 100, 141–149. [Google Scholar] [CrossRef]
- Sulc, M.; Kotikova, Z.; Paznocht, L.; Pivec, V.; Hamouz, K.; Lachman, J. Changes in anthocyanidin levels during the maturation of color-fleshed potato (Solanum tuberosum L.) tubers. Food Chem. 2017, 237, 981–988. [Google Scholar] [CrossRef]
- Qin, Y.; Ma, K.; Liu, P. Effect of potato continuous cropping on genetic diversity of soil microorganisms. Chin. J. Eco-Agric. 2015, 23, 225–232. [Google Scholar]
- Cesarano, G.; Zotti, M.; Antignani, V.; Marra, R.; Scala, F.; Bonanomi, G. Soil sickness and negative plant-soil feedback: A reappraisal of hypotheses. J. Plant Pathol. 2017, 99, 545–570. [Google Scholar]
- Manici, L.M.; Kelderer, M.; Franke-Whittle, I.H.; Ruhmer, T.; Baab, G.; Nicoletti, F.; Caputo, F.; Topp, A.; Insam, H.; Naef, A. Relationship between root-endophytic microbial communities and replant disease in specialized apple growing areas in Europe. Appl. Soil Ecol. 2013, 72, 207–214. [Google Scholar] [CrossRef]
- Kardol, P.; Cornips, N.J.; van Kempen, M.M.L.; Bakx-Schotman, J.M.T.; van der Putten, W.H. Microbe-mediated plant soil feedback causes historical contingency effects in plant community assembly. Ecol. Monogr. 2007, 77, 147–162. [Google Scholar] [CrossRef] [Green Version]
- Van de Voorde, T.F.J.; Ruijten, M.; van der Putten, W.H.; Bezemer, T.M. Can the negative plant-soil feedback of Jacobaea vulgaris be explained by autotoxicity? Basic Appl. Ecol. 2012, 13, 533–541. [Google Scholar] [CrossRef]
- Liu, J.K.; Yan, Z.Q.; Li, X.Z.; Jin, H.; Yang, X.Y.; Xie, M.; Su, A.X.; Qin, B. Characterization of allelochemicals from the rhizosphere soil of Pinellia ternate (Thnub.) and their inhibition activity on protective enzymes. Appl. Soil Ecol. 2018, 125, 301–306. [Google Scholar] [CrossRef]
- Guo, K.; He, X.F.; Yan, Z.Q.; Li, X.Z.; Ren, X.; Pan, L.; Qin, B. Allelochemicals from the Rhizosphere Soil of Cultivated Astragalus hoantchy L. J. Agric. Food Chem. 2016, 64, 3345–3352. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yan, Z.Q.; He, X.F.; Li, X.Z.; Qin, B. Allelochemicals from rhizosphere soils of Glycyrrhiza uralensis Fisch: Discovery of the autotoxic compounds of a traditional herbal medicine. Ind. Crops Prod. 2017, 97, 302–307. [Google Scholar] [CrossRef]
- Xie, M.; Yan, Z.Q.; Ren, X.; Li, X.Z.; Qin, B. Codonopilate A, a triterpenyl ester as main autotoxin in cultivated soil of Codonopsis pilosula (Franch.) Nannf. J. Agric. Food Chem. 2017, 65, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Kato, Y.; Okabe, A.; Itoi, C.; Ooshiro, A.; Kawaide, H.; Natsume, M. Effect of secondary metabolites of tomato (Solanum lycopersicum) on chemotaxis of ralstonia solanacearum, pathogen of bacterial wilt disease. J. Agric. Food Chem. 2019, 67, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Manici, L.M.; Ciavatta, C.; Kelderer, M.; Erschbaumer, G. Replant problems in south tyrol: Role of fungal pathogens and microbial population in conventional and organic apple orchards. Plant Soil. 2003, 256, 315–324. [Google Scholar] [CrossRef]
- Reddy, M.A.; Patrick, Z.A. Colonization of tobacco seedling roots by fluorescent pseudomonad suppressive to black root rot caused by Thielaviopsis basicola. Crop Protection. 1992, 11, 148–154. [Google Scholar] [CrossRef]
- Bonanomi, G.; de Filippis, F.; Cesarano, G.; La Storia, A.; Ercolini, D.; Scala, F. Organic farming induces changes in soil microbiota that affect agro-ecosystem functions. Soil Biol. Biochem. 2016, 103, 327–336. [Google Scholar] [CrossRef]
- Xia, Z.C.; Kong, C.H.; Chen, L.C.; Wang, S.L. Allelochemical-mediated soil microbial community in long-term monospecific Chinese fir forest plantations. Appl. Soil Ecol. 2015, 96, 52–59. [Google Scholar] [CrossRef]
- Dong, L.L.; Xu, J.; Li, Y.; Fang, H.L.; Niu, W.H.; Li, X.W.; Zhang, Y.J.; Ding, W.L.; Chen, S.L. Manipulation of microbial community in the rhizosphere alleviates the replanting issues in Panax ginseng. Soil Biol. Biochem. 2018, 125, 64–74. [Google Scholar] [CrossRef]
- Soltys-Kalina, D.; Murawska, Z.; Strzelczyk-Zyta, D.; Wasilewicz-Flis, I.; Marczewski, W. Phytotoxic potential of cultivated and wild potato species (Solanum sp.): Role of glycoalkaloids, phenolics and flavonoids in phytotoxicity against mustard (Sinapis alba L.). Acta Physiol. Plant. 2019, 41, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Wan, N.X.; Yuan, J.C.; He, W.; Long, W.J.; Zhang, Q.; Zhou, S.M.; Zheng, S.L. Autotoxicity of water extracts from different organs of potato. J. Zhejiang Univ. 2016, 42, 411–418. [Google Scholar]
- Zhang, W.M.; Qiu, H.Z.; Zhang, C.H.; Liu, X.; Gao, Y.A.; Shen, Q.R. Identification and autotoxicity of root exudates of continuous cropping potato at different growth stages. Chin. J. Eco-Agric. 2015, 23, 215–224. [Google Scholar]
- Guo, C.P.; Zi, S.H.; Ou-Yang, C.R.; Wu, B.Z. Effect of aboveground aqueous extracts of maize and potato on the maize growth. J. Maize Sci. 2016, 24, 79–84. [Google Scholar]
- Gamiz, B.; Facenda, G.; Celis, R. Nanoengineered sorbents to increase the persistence of the allelochemical carvone in the rhizosphere. J. Agric. Food Chem. 2019, 67, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Lu, L.Y.; Hu, L.Y.; Cao, W.; Sun, K.; Sun, Q.B.; Siddikee, A.; Shi, R.H.; Dai, C.C. Evidences for the Involvement of auxin, ethylene and ROS signaling during allelochemical benzoic acid-mediated primary root inhibition of Arabidopsis. Plant Cell Physiol. 2018, 59, 1889–1904. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy-Update. Bot. Rev. 1979, 45, 15–109. [Google Scholar] [CrossRef]
- Niro, E.; Marzaioli, R.; de Crescenzo, S.; D’Abrosca, B.; Castaldi, S.; Esposito, A.; Fiorentino, A.; Rutigliano, F.A. Effects of the allelochemical coumarin on plants and soil microbial community. Soil Biol. Biochem. 2016, 95, 30–39. [Google Scholar] [CrossRef]
- Abenavoli, M.R.; Cacco, G.; Sorgona, A.; Marabottini, R.; Paolacci, A.R.; Ciaffi, M.; Badiani, M. The inhibitory effects of coumarin on the germination of durum wheat (Triticum turgidum ssp. durum, cv. Simeto) seeds. J. Chem. Ecol. 2006, 32, 489–506. [Google Scholar] [CrossRef]
- Mushtaq, W.; Ain, Q.; Siddiqui, M.B.; Hakeem, K.R. Cytotoxic allelochemicals induce ultrastructural modifications in Cassia tora L. and mitotic changes in Allium cepa L.: A weed versus weed allelopathy approach. Protoplasma 2019, 256, 857–871. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.F.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Quan, W.X.; Li, C.C.; Qian, C.Y.; Tang, F.H.; Chen, X.J. Allelopathic effects of phenolic acids on seedling growth and photosynthesis in Rhododendron delavayi Franch. Photosynthetica 2019, 57, 377–387. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.D.; Yang, J.S.; Yan, Z.Q.; Sun, Y.H.; Cui, H.Y.; Ding, L.; Qin, B. Allelopathic effect and mechanism of chlorogenic acid on the growth of lettuce. Acta Bot. Boreali-Occident. Sin. 2014, 34, 957–962. [Google Scholar]
- Yin, R.H.; Han, K.; Heller, W.; Albert, A.; Dobrev, P.I.; Zazimalova, E.; Schaffner, A.R. Kaempferol 3-O-rhamnoside-7-O-rhamnoside is an endogenous flavonol inhibitor of polar auxin transport in Arabidopsis shoots. New Phytol. 2014, 20, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.Q.; Yang, W.Y.; Liu, J. Advances on chemical ecology of plant flavonoids. Nat. Prod. Res. Dev. 2018, 30, 2009–2016. [Google Scholar]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterson, E.; Gebbing, T.; Abel, C.; Sim, A.; Telfer, G. Rhizodeposition shapes rhizosphere microbial community structure in organic soil. New Phytol. 2007, 173, 600–610. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Huang, L.F.; Song, L.X.; Xia, X.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Plant-soil feedbacks and soil sickness: From mechanisms to application in agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef]
- Mazzola, M.; Manici, L.M. Apple replant disease: Role of microbial ecology in cause and control. Annu. Rev. Phytopathol. 2012, 50, 45–65. [Google Scholar] [CrossRef]
- Yang, J.I.; Ruegger, P.M.; McKenry, M.V.; Becker, J.O.; Borneman, J. Correlations between root-associated microorganisms and peach replant disease symptoms in a california soil. PLoS ONE 2012, 7, e46420. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; D’Ascoli, R.; Antignani, V.; Capodilupo, M.; Cozzolino, L.; Marzaioli, R.; Puopolo, G.; Rutigliano, F.A.; Scelza, R.; Scotti, R.; et al. Assessing soil quality under intensive cultivation and tree orchards in southern Italy. Appl. Soil Ecol. 2011, 47, 184–194. [Google Scholar] [CrossRef]
- Bertin, C.; Yang, X.H.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil. 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Lin, S.; Dai, L.Q.; Chen, T.; Li, Z.F.; Zhang, Z.Y.; Lin, W.X. Screening and identification of harmful and beneficial microorganisms associated with replanting disease in rhizosphere soil of Pseudostellariae heterophylla. Int. J. Agric. Biol. 2015, 17, 458–466. [Google Scholar] [CrossRef]
- Wei, L.J.; Tan, W.Q.; Wang, G.; Li, Q.; Dong, F.; Guo, Z.Y. The antioxidant and antifungal activity of chitosan derivatives bearing Schiff bases and quaternary ammonium salts. Carbohydr. Polym. 2019, 226, 115256. [Google Scholar] [CrossRef] [PubMed]








| Compounds | Linear Equations a | R2 | Linear Range (ng/mL) | Retention Time (min) | Content (μg/g) |
|---|---|---|---|---|---|
| 1 | y = 0.0093x − 343.9 | 0.9995 | 2000.0–30,000.0 | 23.503 | 1.46 |
| 2 | y = 0.0133x + 123.7 | 0.9998 | 2000.0–30,000.0 | 32.605 | 6.64 |
| 4 | y = 0.0102x + 212.15 | 0.9999 | 500.0–30,000.0 | 10.687 | 0.35 |
| 6 | y = 0.0152x + 518.21 | 0.9993 | 2000.0–30,000.0 | 13.900 | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, A.; Jin, H.; Yang, X.; Guan, J.; Hui, H.; Liu, H.; Cui, Z.; Dun, Z.; Qin, B. Allelochemicals from the Rhizosphere Soil of Potato (Solanum tuberosum L.) and Their Interactions with the Soilborne Pathogens. Plants 2022, 11, 1934. https://doi.org/10.3390/plants11151934
Xin A, Jin H, Yang X, Guan J, Hui H, Liu H, Cui Z, Dun Z, Qin B. Allelochemicals from the Rhizosphere Soil of Potato (Solanum tuberosum L.) and Their Interactions with the Soilborne Pathogens. Plants. 2022; 11(15):1934. https://doi.org/10.3390/plants11151934
Chicago/Turabian StyleXin, Aiyi, Hui Jin, Xiaoyan Yang, Jinfeng Guan, Heping Hui, Haoyue Liu, Zengtuan Cui, Zhiheng Dun, and Bo Qin. 2022. "Allelochemicals from the Rhizosphere Soil of Potato (Solanum tuberosum L.) and Their Interactions with the Soilborne Pathogens" Plants 11, no. 15: 1934. https://doi.org/10.3390/plants11151934