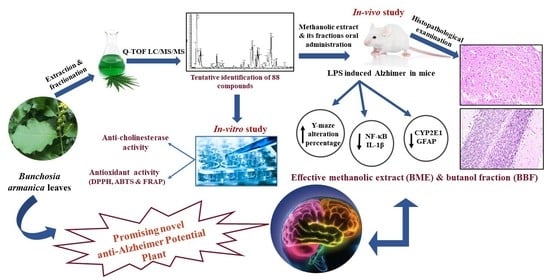
Novel Neuroprotective Potential of Bunchosia armeniaca (Cav.) DC against Lipopolysaccharide Induced Alzheimer’s Disease in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Quantitative Determination of Phenolics and Flavonoids
2.2. Metabolic Profiling Using Q-TOF LC/MS/MS
2.2.1. Flavonoids
Flavonoid Triglycosides
Flavonoid Diglycosides
Flavonoid Monoglycosides
Flavonoid Aglycones
2.2.2. Phenolic Acids
2.2.3. Alkaloids
2.2.4. Polyphenols
2.3. In Vitro Assays
2.3.1. Antioxidant Activity
DPPH Radical Scavenging Assay
ABTS Cation Radical Decolorization Assay
Ferric Reducing Antioxidant Power (FRAP) Assay
2.3.2. Anticholinesterase Activity
2.4. Anti-Alzheimer In Vivo Study
2.4.1. Acute Toxicity
2.4.2. Effects on Behavioral Activity on Y-Maze
2.4.3. Effects on NF-κB and IL-1β
2.4.4. Effects on CYP2E1 and GFAP
2.4.5. Histopathological Examination
3. Materials and Methods
3.1. Plant Material
3.2. Extraction and Fractionation
3.3. Estimation of Total Phenolic and Total Flavonoid Contents
3.4. Metabolic Profiling Using Q-TOF LC/MS/MS
3.4.1. Sample preparation
3.4.2. Q-TOF LC/MS/MS Analysis
3.4.3. Mass Spectrophotometry
3.5. In Vitro Antioxidant Assays
3.5.1. DPPH Radical Scavenging Assay
3.5.2. ABTS Cation Radical Decolorization Assay
3.5.3. Ferric Reducing Antioxidant Power Assay
3.6. In Vitro Acetylcholinesterase Inhibition Activity
3.7. In Vivo Study
3.7.1. Acute Toxicity Study
3.7.2. Evaluation of Anti-Alzheimer Activity
Animals
Chemicals and Kits
Experimental Design
Effects on Y-Maze in LPS-Induced Alzheimer in Mice
Tissue Biochemical Analysis
Histopathological Examination
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Queiroz, G.S.; Heller, M.; Arruda-Silva, F.; Nascimento, M.V.; Micke, G.A.; Dalmarco, E.M.; Pizzolatti, M.G.; Brighente, I.M. Antibacterial and Anti-Inflammatory activities of Bunchosia armeniaca (Cav.) DC.(Malpighiaceae). Rec. Nat. Prod. 2015, 9, 419–431. [Google Scholar]
- Karunasena, G.; Chandrajith, V.; Nawaratne, S. Physicochemical Characteristics of Pea Nut Butter Fruit (Bunchosia armeniaca). Int. J. Food Sci. Nutr. 2018, 3, 46–51. [Google Scholar]
- Lorenzi, H.; LaRocca, L.L. Brazilian Fruits & Cultivated Exotics (for Consuming in Natura); Instituto Plantarum de Estudos da Flora: Nova Odessa, Brazil, 2006. [Google Scholar]
- Allen, P.H. Book 2, Field Notes; Smithsonian Institution Smithsonian Institution Archives: Panama, Colombia.
- Karunasena, G.; Chandrajith, V.; Navaratne, S. Antioxidant capacity and total phenol content of peanut butter fruit (Bunchosia armenica). J. Pharmacogn. Phytochem. 2018, 7, 343–346. [Google Scholar]
- Giraldi, M.; Hanazaki, N. Uso e conhecimento tradicional de plantas medicinais no Sertão do Ribeirão, Florianópolis, SC, Brasil. Acta Bot. Bras. 2010, 24, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Premathilaka, R.; Silva, M. Bioactive Compounds and Antioxidant Activity of Bunchosia armenica. World J. Pharm. Pharm. Sci. 2016, 5, 1237–1247. [Google Scholar] [CrossRef]
- Queiroz, G.S. Flavonoides de Bunchosia Armeniaca e Derivados de 2-Arilideno-1-a-Tetralona: Obtenção e Atividades Biológicas; Federal University of Santa Catarina Center for Physical and Mathematical Science: Florianópolis, Brazil, 2012. [Google Scholar]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [Green Version]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones, flavanones, and human health: Epidemiological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, I. Defining Alzheimer as a common age-related neurodegenerative process not inevitably leading to dementia. Prog. Neurobiol. 2012, 97, 38–51. [Google Scholar] [CrossRef]
- Peters, A. The effects of normal aging on myelin and nerve fibers: A review. J. Neurocytol. 2002, 31, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Association, A.s. 2022 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2022, 18, 700–789. [Google Scholar] [CrossRef]
- Vecchio, I.; Sorrentino, L.; Paoletti, A.; Marra, R.; Arbitrio, M. The State of The Art on Acetylcholinesterase Inhibitors in the Treatment of Alzheimer’s Disease. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211029113. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Stanciu, G.D.; Luca, A.; Rusu, R.N.; Bild, V.; Beschea Chiriac, S.I.; Solcan, C.; Bild, W.; Ababei, D.C. Alzheimer’s disease pharmacotherapy in relation to cholinergic system involvement. Biomolecules 2020, 10, 40. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.; Raimundo, A.F.; Menezes, R.; Martins, I.C. Islet amyloid polypeptide & amyloid beta peptide roles in Alzheimer’s disease: Two triggers, one disease. Neural Regen. Res. 2021, 16, 1127–1130. [Google Scholar] [CrossRef]
- Chong, F.P.; Ng, K.Y.; Koh, R.Y.; Chye, S.M. Tau proteins and tauopathies in Alzheimer’s disease. Cell. Mol. Neurobiol. 2018, 38, 965–980. [Google Scholar] [CrossRef]
- Leblhuber, F.; Ehrlich, D.; Steiner, K.; Geisler, S.; Fuchs, D.; Lanser, L.; Kurz, K. The Immunopathogenesis of Alzheimer’s Disease Is Related to the Composition of Gut Microbiota. Nutrients 2021, 13, 361. [Google Scholar] [CrossRef]
- Devi, S.; Kumar, V.; Singh, S.K.; Dubey, A.K.; Kim, J.-J. Flavonoids: Potential candidates for the treatment of neurodegenerative disorders. Biomedicines 2021, 9, 99. [Google Scholar] [CrossRef]
- Suß, P.; Lana, A.J.; Schlachetzki, J.C. Chronic peripheral inflammation: A possible contributor to neurodegenerative diseases. Neural Regen. Res. 2021, 16, 1711–1714. [Google Scholar] [CrossRef]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a common feature of neurodegenerative disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huat, T.J.; Camats-Perna, J.; Newcombe, E.A.; Valmas, N.; Kitazawa, M.; Medeiros, R. Metal toxicity links to Alzheimer’s disease and neuroinflammation. J. Mol. Biol. 2019, 431, 1843–1868. [Google Scholar] [CrossRef] [PubMed]
- Mouzat, K.; Chudinova, A.; Polge, A.; Kantar, J.; Camu, W.; Raoul, C.; Lumbroso, S. Regulation of brain cholesterol: What role do liver X receptors play in neurodegenerative diseases? Int. J. Mol. Sci. 2019, 20, 3858. [Google Scholar] [CrossRef] [Green Version]
- Vinuesa, A.; Pomilio, C.; Gregosa, A.; Bentivegna, M.; Presa, J.; Bellotto, M.; Saravia, F.; Beauquis, J. Inflammation and Insulin Resistance as Risk Factors and Potential Therapeutic Targets for Alzheimer’s Disease. Front. Neurosci. 2021, 15, 653651. [Google Scholar] [CrossRef] [PubMed]
- Biesmans, S.; Meert, T.F.; Bouwknecht, J.A.; Acton, P.D.; Davoodi, N.; De Haes, P.; Kuijlaars, J.; Langlois, X.; Matthews, L.J.; Ver Donck, L. Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediat. Inflamm. 2013, 2013, 271359. [Google Scholar] [CrossRef]
- Mahdi, O.; Baharuldin, M.T.H.; Nor, N.H.M.; Chiroma, S.M.; Jagadeesan, S.; Moklas, M.A.M. Chemicals used for the induction of Alzheimer’s disease-like cognitive dysfunctions in rodents. Biomed. Res. Ther. 2019, 6, 3460–3484. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, R.; Wan Yaacob, W.; Othman, Z.; Long, I.; Ahmad, A.; Al-Rahbi, B. Lipopolysaccharide-induced memory impairment in rats: A model of Alzheimer’s disease. Physiol. Res. 2017, 66, 553–565. [Google Scholar] [CrossRef]
- Li, S.; Li, S.-K.; Gan, R.-Y.; Song, F.-L.; Kuang, L.; Li, H.-B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crops Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Blank, D.E.; Justen, D.; Fraga, S.; Peixoto, C.R.; de Moura, N.F. Chemical Composition and Antioxidant Activity of Bunchosia glandulifera Fruit at Different Ripening Stages. Food Nutr. Sci. 2018, 9, 1147–1159. [Google Scholar] [CrossRef] [Green Version]
- De Vos, R.C.; Moco, S.; Lommen, A.; Keurentjes, J.J.; Bino, R.J.; Hall, R.D. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778–791. [Google Scholar] [CrossRef]
- El Sayed, A.M.; Basam, S.M.; Marzouk, H.S.; El-Hawary, S. LC–MS/MS and GC–MS profiling as well as the antimicrobial effect of leaves of selected Yucca species introduced to Egypt. Sci. Rep. 2020, 10, 17778. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Samiotaki, M.; Panayotou, G.; Oreopoulou, V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules 2007, 12, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Khan, R.A.; Abdel-Hafez, A.A.; Abdel-Aziz, M.; Ahmed, E.; Enany, S.; Mahgoub, S.; Al-Rugaie, O.; Alsharidah, M.; Aly, M.S. Phytochemical profiling, in vitro and in silico anti-microbial and anti-cancer activity evaluations and Staph GyraseB and h-TOP-IIβ receptor-docking studies of major constituents of Zygophyllum coccineum L. Aqueous-ethanolic extract and its subsequent fractions: An approach to validate traditional phytomedicinal knowledge. Molecules 2021, 26, 577. [Google Scholar] [CrossRef] [PubMed]
- March, R.E.; Miao, X.S.; Metcalfe, C.D. A fragmentation study of a flavone triglycoside, kaempferol-3-O-robinoside-7-O-rhamnoside. Rapid Commun. Mass Spectrom. 2004, 18, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Torras-Claveria, L.; Jáuregui, O.; Codina, C.; Tiburcio, A.F.; Bastida, J.; Viladomat, F. Analysis of phenolic compounds by high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry in senescent and water-stressed tobacco. Plant Sci. 2012, 182, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Müller, H.; Müller, A.; Karar, M.G.E.; Kuhnert, N. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L.(Caprifoliaceae) leaves by LC–MSn. Phytochemistry 2014, 108, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Salerno, R.; Casale, F.; Calandruccio, C.; Procopio, A. Characterization of flavonoids in Citrus bergamia (Bergamot) polyphenolic fraction by liquid chromatography–high resolution mass spectrometry (LC/HRMS). Pharma. Nutr. 2016, 4, S1–S7. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Guo, S.; Wang, Z.; Jiang, L.; Wang, F.; Zhang, J.; Liu, B. Profiling and comparison of the metabolites of diosmetin and diosmin in rat urine, plasma and feces using UHPLC-LTQ-Orbitrap MSn. J. Chromatogr. B 2019, 1124, 58–71. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-M.; Li, C.-H.; Zhu, X.-R.; Deng, Y.-M.; Sun, W.; Wang, L.-S.; Chen, F.-D.; Zhang, Z. The identification of flavonoids and the expression of genes of anthocyanin biosynthesis in the chrysanthemum flowers. Biol. Plant. 2012, 56, 458–464. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Wang, W.; Sooranna, S.R.; Shi, Y.; Chen, Y.; Wu, C.; Zeng, J.; Tang, Q.; Xie, H. Chemical profiles and simultaneous quantification of Aurantii fructus by use of HPLC-Q-TOF-MS combined with GC-MS and HPLC methods. Molecules 2018, 23, 2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueirinha, A.; Paranhos, A.; Pérez-Alonso, J.J.; Santos-Buelga, C.; Batista, M.T. Cymbopogon citratus leaves: Characterization of flavonoids by HPLC–PDA–ESI/MS/MS and an approach to their potential as a source of bioactive polyphenols. Food Chem. 2008, 110, 718–728. [Google Scholar] [CrossRef]
- Hassan, W.H.; Abdelaziz, S.; Al Yousef, H.M. Chemical composition and biological activities of the aqueous fraction of Parkinsonea aculeata L. growing in Saudi Arabia. Arab. J. Chem. 2019, 12, 377–387. [Google Scholar] [CrossRef]
- Mahrous, E.A.; Elosaily, A.H.; Salama, A.A.; Salama, A.M.; El-Zalabani, S.M. Oral and Topical Anti-Inflammatory Activity of Jatropha integerrima Leaves Extract in Relation to Its Metabolite Profile. Plants 2022, 11, 218. [Google Scholar] [CrossRef]
- Zeng, X.; Su, W.; Zheng, Y.; Liu, H.; Li, P.; Zhang, W.; Liang, Y.; Bai, Y.; Peng, W.; Yao, H. Uflc-q-tof-ms/ms-based screening and identification of flavonoids and derived metabolites in human urine after oral administration of exocarpium citri grandis extract. Molecules 2018, 23, 895. [Google Scholar] [CrossRef] [Green Version]
- Gómez, J.D.; Vital, C.E.; Oliveira, M.G.; Ramos, H.J. Broad range flavonoid profiling by LC/MS of soybean genotypes contrasting for resistance to Anticarsia gemmatalis (Lepidoptera: Noctuidae). PLoS ONE 2018, 13, e0205010. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Wen, J.H.; Li, P.; Gao, W.; Yang, H. Target profiling of flavonol glycosides in the extract of Ginkgo biloba leaf and their pharmacokinetics in rat plasma by ultra-high-performance liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2022, 45, 728–738. [Google Scholar] [CrossRef]
- Attallah, N.G.; Negm, W.A.; Elekhnawy, E.; Elmongy, E.I.; Altwaijry, N.; El-Haroun, H.; El-Masry, T.A.; El-Sherbeni, S.A. Elucidation of phytochemical content of Cupressus macrocarpa leaves: In vitro and in vivo antibacterial effect against methicillin-resistant Staphylococcus aureus clinical isolates. Antibiotics 2021, 10, 890. [Google Scholar] [CrossRef]
- El-Shiekh, R.A.; Ashour, R.M.; Abd El-Haleim, E.A.; Ahmed, K.A.; Abdel-Sattar, E. Hibiscus sabdariffa L.: A potent natural neuroprotective agent for the prevention of streptozotocin-induced Alzheimer’s disease in mice. Biomed. Pharmacother. 2020, 128, 110303. [Google Scholar] [CrossRef]
- Saber, F.R.; Mohsen, E.; El-Hawary, S.; Eltanany, B.M.; Elimam, H.; Sobeh, M.; Elmotayam, A.K. Chemometric-enhanced metabolic profiling of five Pinus species using HPLC-MS/MS spectrometry: Correlation to in vitro anti-aging, anti-Alzheimer and antidiabetic activities. J. Chromatogr. B 2021, 1177, 122759. [Google Scholar] [CrossRef]
- Saibabu, V.; Fatima, Z.; Khan, L.A.; Hameed, S. Therapeutic potential of dietary phenolic acids. Adv. Pharmacol. Sci. 2015, 2015, 823539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lv, H.; Li, Z.; Jiang, K.; Lee, M.R. HPLC/QTOF-MS/MS application to investigate phenolic constituents from Ficus pandurata H. aerial roots. Biomed. Chromatogr. 2015, 29, 860–868. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Lozano-Castellón, J.; Mardones, C.; Pérez, A.J.; Saéz, V.; Riquelme, S.; von Baer, D.; Vallverdú-Queralt, A. Phenolic Profile of Grape Canes: Novel Compounds Identified by LC-ESI-LTQ-Orbitrap-MS. Molecules 2019, 24, 3763. [Google Scholar] [CrossRef] [Green Version]
- Kowmudi, G.; Nagappan, K.; Anoop, K.; Sailaja, M.; ST, N. A validated LC-MS/MS method for the quantification of trigonelline in marketed dietary supplements. Curr. Bioact. Compd. 2020, 16, 687–695. [Google Scholar] [CrossRef]
- Zhao, T.; Zheng, S.-S.; Zhang, B.-F.; Li, Y.-Y.; Bligh, S.A.; Wang, C.-H.; Wang, Z.-T. Metabolic pathways of the psychotropic-carboline alkaloids, harmaline and harmine, by liquid chromatography/mass spectrometry and NMR spectroscopy. Food Chem. 2012, 134, 1096–1105. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Mondal, G.; Butawan, M.; Bloomer, R.J.; Yates, C.R. Development of a liquid chromatography-tandem mass spectrometry (LC–MS/MS) method for characterizing caffeine, methylliberine, and theacrine pharmacokinetics in humans. J. Chromatogr. B 2020, 1155, 122278. [Google Scholar] [CrossRef]
- Silva, S.d.F.; Blank, D.E.; Peixoto, C.R.; de Jesus da Silveira Moreira, J.; Fernandes de Moura, N. Bioactive compounds and antioxidant activity of Bunchosia glandulifera. Int. J. Food Prop. 2016, 19, 467–473. [Google Scholar] [CrossRef]
- Russo, H.M.; Queiroz, E.F.; Marcourt, L.; Rutz, A.; Allard, P.-M.; de Almeida, R.F.; Carvalho, N.M.; Wolfender, J.-L.; da Silva Bolzani, V. Phytochemical analysis of the methanolic leaves extract of Niedenzuella multiglandulosa (Malpighiaceae), a plant species toxic to cattle in Brazil. Phytochem. Lett. 2020, 37, 10–16. [Google Scholar] [CrossRef]
- Gouvêa, A.C.M.S.; Araujo, M.C.P.d.; Schulz, D.F.; Pacheco, S.; Godoy, R.L.d.O.; Cabral, L.M.C. Anthocyanins standards (cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside) isolation from freeze-dried açaí (Euterpe oleraceae Mart.) by HPLC. Food Sci. Technol. 2012, 32, 43–46. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, R.F.; Valls-Fonayet, J.; Richard, T.; Cantos-Villar, E. A rapid quantification of stilbene content in wine by ultra-high pressure liquid chromatography–Mass spectrometry. Food Control 2020, 108, 106821. [Google Scholar] [CrossRef]
- Yuzuak, S.; Ballington, J.; Xie, D.-Y. HPLC-qTOF-MS/MS-based profiling of flavan-3-ols and dimeric proanthocyanidins in berries of two muscadine grape hybrids FLH 13-11 and FLH 17-66. Metabolites 2018, 8, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Zhan, J.; Liu, X.-L.; Wang, Y.; Ji, J.; He, Q.-Q. Dietary flavonoids intake and risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Clin. Nutr. 2014, 33, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef]
- Min, H.-Y.; Jang, H.-J.; Park, K.H.; Hyun, S.Y.; Park, S.J.; Kim, J.H.; Son, J.; Kang, S.S.; Lee, H.-Y. The natural compound gracillin exerts potent antitumor activity by targeting mitochondrial complex II. Cell Death Dis. 2019, 10, 810. [Google Scholar] [CrossRef] [Green Version]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Nadeem, M.; Mumtaz, M.W.; Danish, M.; Rashid, U.; Mukhtar, H.; Irfan, A.; Anwar, F.; Saari, N. UHPLC-QTOF-MS/MS metabolites profiling and antioxidant/antidiabetic attributes of Cuscuta reflexa grown on Casearia tomentosa: Exploring phytochemicals role via molecular docking. Int. J. Food Prop. 2020, 23, 918–940. [Google Scholar] [CrossRef]
- Juhaimi, F.Y.A. Antioxidant and antifungal activity of some aromatic plant extracts. J. Med. Plants Res. 2011, 5, 1361–1366. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- El Mannoubi, I. Effect of extraction solvent on phenolic composition, antioxidant and antibacterial activities of skin and pulp of Tunisian red and yellow–orange Opuntia Ficus Indica fruits. J. Food Meas. Charact. 2021, 15, 643–651. [Google Scholar] [CrossRef]
- Taslimi, P.; Köksal, E.; Gören, A.C.; Bursal, E.; Aras, A.; Kılıç, Ö.; Alwasel, S.; Gülçin, İ. Anti-Alzheimer, antidiabetic and antioxidant potential of Satureja cuneifolia and analysis of its phenolic contents by LC-MS/MS. Arab. J. Chem. 2020, 13, 4528–4537. [Google Scholar] [CrossRef]
- Mosquera, O.M.; Correra, Y.M.; Niño, J. Antioxidant activity of plant extracts from Colombian flora. Rev. Bras. Farmacogn. 2009, 19, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Ouerghemmi, I.; Harbeoui, H.; Aidi Wannes, W.; Bettaieb Rebey, I.; Hammami, M.; Marzouk, B.; Saidani Tounsi, M. Phytochemical composition and antioxidant activity of Tunisian cactus pear (Opuntia ficus indica L.) flower. J. Food Biochem. 2017, 41, e12390. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muñiz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- Sandhar, H.K.; Kumar, B.; Prasher, S.; Tiwari, P.; Salhan, M.; Sharma, P. A review of phytochemistry and pharmacology of flavonoids. Int. Pharm. Sci. 2011, 1, 25–41. [Google Scholar]
- Alirezalu, A.; Salehi, P.; Ahmadi, N.; Sonboli, A.; Aceto, S.; Hatami Maleki, H.; Ayyari, M. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of Iran. Int. J. Food Prop. 2018, 21, 452–470. [Google Scholar] [CrossRef] [Green Version]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crops Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflammation 2008, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Ali, T.; Park, H.Y.; Badshah, H.; Rehman, S.U.; Kim, M.O. Neuroprotective effect of fisetin against amyloid-beta-induced cognitive/synaptic dysfunction, neuroinflammation, and neurodegeneration in adult mice. Mol. Neurobiol. 2017, 54, 2269–2285. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, F.; Sha, L.; Wang, S.; Tao, L.; Yao, L.; He, M.; Yao, Z.; Liu, H.; Zhu, Z. (−)-Epigallocatechin-3-gallate ameliorates learning and memory deficits by adjusting the balance of TrkA/p75NTR signaling in APP/PS1 transgenic mice. Mol. Neurobiol. 2014, 49, 1350–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, D.; Gopinath, K.; Sudhandiran, G. Fisetin enhances behavioral performances and attenuates reactive gliosis and inflammation during aluminum chloride-induced neurotoxicity. Neuromol. Med. 2013, 15, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Sonya, V.; Nader, A.G.; Calogero, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 2011, 44, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer’s disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef]
- Sriraksa, N.; Wattanathorn, J.; Muchimapura, S.; Tiamkao, S.; Brown, K.; Chaisiwamongkol, K. Cognitive-enhancing effect of quercetin in a rat model of Parkinson’s disease induced by 6-hydroxydopamine. Evid. Based Complement. Alternat. Med. 2012, 2012, 823206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashrafpour, M.; Parsaei, S.; Sepehri, H. Quercetin improved spatial memory dysfunctions in rat model of intracerebroventricular streptozotocin-induced sporadic Alzheimer’s disease. Natl. J. Physiol. Pharm. Pharmacol. 2015, 5, 411–415. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Ali, T.; Rehman, S.U.; Khan, M.S.; Alam, S.I.; Ikram, M.; Muhammad, T.; Saeed, K.; Badshah, H.; Kim, M.O. Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front. Pharmacol. 2018, 9, 1383. [Google Scholar] [CrossRef] [PubMed]
- Galvão, S.; Marques, L.; Oliveira, M.; Carlini, E. Heteropterys aphrodisiaca (extract BST0298): A Brazilian plant that improves memory in aged rats. J. Ethnopharmacol. 2002, 79, 305–311. [Google Scholar] [CrossRef]
- Badshah, H.; Ali, T.; Rehman, S.-u.; Amin, F.-u.; Ullah, F.; Kim, T.H.; Kim, M.O. Protective effect of lupeol against lipopolysaccharide-induced neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain. J. Neuroimmune Pharmacol. 2016, 11, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Tajuddin, N.; Moon, K.-H.; Marshall, S.A.; Nixon, K.; Neafsey, E.J.; Kim, H.-Y.; Collins, M.A. Neuroinflammation and neurodegeneration in adult rat brain from binge ethanol exposure: Abrogation by docosahexaenoic acid. PLoS ONE 2014, 9, e101223. [Google Scholar] [CrossRef] [Green Version]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, F.; Metz, C.N.; Bucala, R.; Lesur, O. Endotoxin-induced myocardial dysfunction: Effects of macrophage migration inhibitory factor neutralization. Circ. Res. 2005, 96, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pålsson-McDermott, E.M.; O’Neill, L.A. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef]
- Dourado, N.S.; Souza, C.d.S.; De Almeida, M.M.A.; Bispo da Silva, A.; Dos Santos, B.L.; Silva, V.D.A.; De Assis, A.M.; da Silva, J.S.; Souza, D.O.; Costa, M.d.F.D. Neuroimmunomodulatory and neuroprotective effects of the flavonoid apigenin in in vitro models of neuroinflammation associated with Alzheimer’s disease. Front. Aging Neurosci. 2020, 12, 119. [Google Scholar] [CrossRef]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear factor-κB: Its role in health and disease. J. Mol. Med. 2004, 82, 434–448. [Google Scholar] [CrossRef]
- Afifi, N.; Ramadan, A.; El-Eraky, W.; Salama, A.; El-Fadaly, A.; Hassan, A. Quercetin protects against thioacetamide induced hepatotoxicity in rats through decreased oxidative stress biomarkers, the inflammatory cytokines;(TNF-α),(NF-κ B) and DNA fragmentation. Der. Pharma. Chem. 2016, 8, 48–55. [Google Scholar]
- Chen, P.; Huo, X.; Liu, W.; Li, K.; Sun, Z.; Tian, J. Apigenin exhibits anti-inflammatory effects in LPS-stimulated BV2 microglia through activating GSK3β/Nrf2 signaling pathway. Immunopharmacol. Immunotoxicol. 2020, 42, 9–16. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Fang, S.-C.; Huang, H.-W.; Yen, G.-C. Anti-inflammatory effects of triterpenes and steroid compounds isolated from the stem bark of Hiptage benghalensis. J. Funct. Foods 2015, 12, 420–427. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempuraj, D.; Thangavel, R.; Selvakumar, G.P.; Zaheer, S.; Ahmed, M.E.; Raikwar, S.P.; Zahoor, H.; Saeed, D.; Natteru, P.A.; Iyer, S. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front. Cell. Neurosci. 2017, 11, 216. [Google Scholar] [CrossRef]
- Cederbaum, A.I.; Yang, L.; Wang, X.; Wu, D. CYP2E1 sensitizes the liver to LPS-and TNF α-induced toxicity via elevated oxidative and nitrosative stress and activation of ASK-1 and JNK mitogen-activated kinases. Int. J. Hepatol. 2012, 2012, 582790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisniewski, H.; Wegiel, J.; Wang, K.; Lach, B. Ultrastructural studies of the cells forming amyloid in the cortical vessel wall in Alzheimer’s disease. Acta Neuropathol. 1992, 84, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Korolainen, M.A.; Auriola, S.; Nyman, T.A.; Alafuzoff, I.; Pirttilä, T. Proteomic analysis of glial fibrillary acidic protein in Alzheimer’s disease and aging brain. Neurobiol. Dis. 2005, 20, 858–870. [Google Scholar] [CrossRef]
- KamalpreetKaura, N.K.; Sharmaa, N. Phytochemicals as future drugs for Parkinson’s disease: A Review. Plant Arch. 2021, 21, 2338–2349. [Google Scholar] [CrossRef]
- Syarifah-Noratiqah, S.-B.; Naina-Mohamed, I.; Zulfarina, M.S.; Qodriyah, H. Natural polyphenols in the treatment of Alzheimer’s disease. Curr. Drug Targets 2018, 19, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Jugder, B.-E.; Poljak, A.; Jayasena, T.; Mansour, H.; Mohammad Nabavi, S.; Sachdev, P.; Grant, R. Resveratrol as a potential therapeutic candidate for the treatment and management of Alzheimer’s disease. Curr. Top. Med. Chem. 2016, 16, 1951–1960. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.-y.; Sun, Y.; Wang, H.; Shan, H.; Wang, S. Baicalin protects LPS-induced blood–brain barrier damage and activates Nrf2-mediated antioxidant stress pathway. Int. Immunopharmacol. 2021, 96, 107725. [Google Scholar] [CrossRef]
- Malar, D.S.; Prasanth, M.I.; Jeyakumar, M.; Balamurugan, K.; Devi, K.P. Vitexin prevents Aβ proteotoxicity in transgenic Caenorhabditis elegans model of Alzheimer’s disease by modulating unfolded protein response. J. Biochem. Mol. Toxicol. 2021, 35, e22632. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, J.; Hua, L.; Han, B.; Zhang, Y.; Yang, X.; Zeng, Z.; Bai, H.; Yin, H. Effects of caffeic acid on learning deficits in a model of Alzheimer’s disease. Int. J. Mol. Med. 2016, 38, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.R.; Kim, J.H.; Lee, S.; Cho, E.J.; Kim, H.Y. Protective effects of protocatechuic acid against cognitive impairment in an amyloid beta-induced Alzheimer’s disease mouse model. Food Chem. Toxicol. 2020, 144, 111571. [Google Scholar] [CrossRef]
- Attard, E. A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Kiranmai, M.; Kumar, C.M.; Mohammed, I. Comparison of total flavanoid content of Azadirachta indica root bark extracts prepared by different methods of extraction. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 254–261. [Google Scholar]
- Fayek, N.M.; Farag, M.A.; Abdel Monem, A.R.; Moussa, M.Y.; Abd-Elwahab, S.M.; El-Tanbouly, N.D. Comparative metabolite profiling of four citrus peel cultivars via ultra-performance liquid chromatography coupled with quadrupole-time-of-flight-mass spectrometry and multivariate data analyses. J. Chromatogr. Sci. 2019, 57, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Boly, R.; Lamkami, T.; Lompo, M.; Dubois, J.; Guissou, I. DPPH free radical scavenging activity of two extracts from Agelanthus dodoneifolius (Loranthaceae) leaves. Int. J. Toxicol. Pharmacol. Res. 2016, 8, 29–34. [Google Scholar]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Moein, M.; Moein, S.; Farmani, F.; Rozbehan, S.; Sabahi, Z. Examination the antioxidant potentials and antidiabetic properties of phenolic extracts of some Iranian honeys. J. Nephropharmacol. 2021, 11, e06. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres Jr, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bensari, S.; Ouelbani, R.; Yilmaz, M.A.; Bensouici, C.; Gokalp, E.; Khelifi, D. Phytochemical profiles of Iris unguicularis Poir. with antioxidant, antibacterial, and anti-Alzheimer activities. Acta Sci. Nat. 2020, 7, 74–87. [Google Scholar] [CrossRef]
- El Kady, W.M.; Salama, A.A.; Desoukey, S.Y.; Hagag, E.G.; El-Shenawy, S.M.; El-Shanawany, M. Comparative DNA profiling, botanical identification and biological evaluation of Gazania longiscapa DC and Gazania rigens L. Bull. Fac. Pharm. Cairo Univ. 2015, 53, 129–145. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.E.; Hwang, C.J.; Lee, H.P.; Kim, C.S.; Son, D.J.; Ham, Y.W.; Hellström, M.; Han, S.-B.; Kim, H.S.; Park, E.K. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology 2017, 117, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, N.; Suemaru, K.; Takechi, K.; Li, B.; Araki, H. Inhibitory effects of valproate on impairment of Y-maze alternation behavior induced by repeated electroconvulsive seizures and c-Fos protein levels in rat brains. Acta Med. Okayama 2011, 65, 269–277. [Google Scholar] [CrossRef]
- Hughes, R.N. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 2004, 28, 497–505. [Google Scholar] [CrossRef]
- Miwa, M.; Tsuboi, M.; Noguchi, Y.; Enokishima, A.; Nabeshima, T.; Hiramatsu, M. Effects of betaine on lipopolysaccharide-induced memory impairment in mice and the involvement of GABA transporter 2. J. Neuroinflamm. 2011, 8, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salama, A.; Hegazy, R.; Hassan, A. Intranasal chromium induces acute brain and lung injuries in rats: Assessment of different potential hazardous effects of environmental and occupational exposure to chromium and introduction of a novel pharmacological and toxicological animal model. PLoS ONE 2016, 11, e0168688. [Google Scholar] [CrossRef]
- Salama, A.; Fayed, H.M.; Elgohary, R. L-carnitine alleviated acute lung injuries induced by potassium dichromate in rats: Involvement of Nrf2/HO-1 signaling pathway. Heliyon 2021, 7, e07207. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier Health Sciences: London, UK, 2018. [Google Scholar]
- Chen, Z.; Bertin, R.; Froldi, G. EC50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef]










| No. | RT (min) | Mol. Ion m/z | Identified Compound | Molecular Formula | Error (ppm) | Fragment Ions | |
|---|---|---|---|---|---|---|---|
| [M + H]+ [M]+ | [M − H]− [M]− | ||||||
| Amino acids | |||||||
| 1 | 0.98 | 163.1108 | 5-Hydroxylysine | C6H14N2O3 | 2.8 | 145.1001, 128.0689, 117.0568, 100.0812 | |
| 2 | 1.16 | 156.0502 | L-Histidine | C6H9N3O2 | 0.7 | 110.0036, 93.0434, 83.0586, 68.9831, 66.0199 | |
| 6 | 1.21 | 102.0549 | 3-Aminoisobutyric acid | C4H9NO2 | 0.6 | Not fragmented | |
| 7 | 1.22 | 146.0448 | L-Glutamic acid | C5H9NO4 | 0.8 | 128.0347, 102.0588, 100.0358, 91.0552, 72.0092 | |
| 9 | 1.27 | 104.1065 | 2-Aminoisobutyric acid | C4H9NO2 | 1 | 87.0409, 60.0787, 58.0643, 56.0483 | |
| 11 | 1.34 | 205.0677 | 203.0818 | L-Tryptophan | C11H12N2O2 | 3 | 188.0687, 170.0324, 159.0884, 144.0814, 142.0646, 132.0801, 130.0656, 118.0644, 74.0168 |
| 14 | 1.41 | 147.0665 | L-Glutamine | C5H10N2O3 | −1.2 | 130.0494, 129.0208, 84.0424, 56.0485, 55.0151 | |
| 18 | 1.52 | 130.0860 | L-Hydroxyproline | C5H9NO3 | 6.6 | 113.04095 | |
| 19 | 1.56 | 182.0796 | Tyrosine | C9H11NO3 | 4.7 | 147.0424, 136.0729, 123.0443, 119.0515, 95.0484, 91.0518, 77.03807 | |
| 20 | 1.76 | 130.0493 | L-Pyroglutamic acid | C5H7NO3 | 1 | 112.1001, 84.08121, 70.06413, 56.05059 | |
| 21 | 1.85 | 132.1025 | D-Allo-isoleucine | C6H13NO2 | −4.8 | 86.0952, 69.0702, 57.0554 | |
| 22 | 2.15 | 166.0860 | Phenylalanine | C9H11NO2 | 0.4 | 131.0498, 120.0799, 103.0523, 91.0546, 77.0381 | |
| 23 | 2.16 | 182.0818 | L-Methionine sulfone | C5H11NO4S | −1.4 | 136.0399, 56.0465 | |
| Alkaloids | |||||||
| 13 | 1.39 | 138.0551 | Trigonelline | C7H7NO2 | −0.6 | 110.0599, 94.0651, 92.0489, 78.8031 | |
| 17 | 1.51 | 215.1401 | Harmaline | C13H14N2O | −2.5 | 200.1342, 169.1384, 156.0641, 70.0640 | |
| 28 | 3.37 | 195.1122 | Caffeine | C8H10N4O2 | 3.3 | 163.0349, 138.0005, 95.0816, 70.0642 | |
| Phenolic acids | |||||||
| 12 | 1.35 | 179.0549 | Caffeic acid | C9H8O4 | 3.5 | 161.0414, 135.0395, 133.0644, 117.0399, 109.0569 | |
| 15 | 1.43 | 355.1033 | 353.0872 | Chlorogenic acid | C16H18O9 | 1.1 | 284.0475, 191.0546, 179.0548, 173.0496 |
| 16 | 1.45 | 153.0263 | Protocatechuic acid | C7H6O4 | −3.9 | 135.0166, 112.9867, 109.0180, 84.9922, 78.95688 | |
| 25 | 2.33 | 154.0476 | 3-Hydroxyanthranilic acid | C7H7NO3 | 10.9 | 136.0369, 108.0399, 81.0658, 80.0468, 53.0369 | |
| 29 | 3.40 | 139.0376 | 137.0236 | 4-Hydroxybenzoic acid | C7H6O3 | 3.1 | 121.0227, 111.0877, 95.0595, 67.0373 |
| 31 | 3.97 | 183.1393 | 3,4-Dihydroxymandelic acid | C8H8O5 | −0.4 | Not fragmented | |
| Flavonoid triglycosides | |||||||
| 47 | 6.26 | 741.2262 | 739.2115 | Robinin (Kaempferol-3-O-robinoside-7-O-rhamnoside) | C33H40O19 | −0.6 | 723.23969, 595.1675, 449.10574, 433.1141, 287.0566, 147.0656, 129.0527, 71. 0485 |
| Flavonoid diglycosides | |||||||
| 32 | 4.02 | 595.1846 | Saponarin (Apigenin-6-C-glucoside -7-O-glucoside) | C27H30O15 | −7.4 | Not fragmented | |
| 34 | 4.74 | 593.1525 | Kaempferol-7-O-neohesperidoside | C27H30O15 | −1.5 | 503.12836, 473.0619, 431.09586, 285.0508 | |
| 38 | 5.20 | 593.2739 | 591.2206 | Acaciin (Acacetin 7-O-rutinoside) | C28H32O14 | 1.5 | 575.5092, 503.2012, 473.22516, 447.2165, 431.18842, 285.1368, 267.12045 |
| 52 | 6.66 | 609.1923 | Diosmin (Diosmetin 7-O-rutinoside) | C28H32O15 | 0.4 | 591.2541, 549.2500, 463.0887, 447.2338, 331.2256, 301.2999, 184.0679 | |
| 57 | 7.03 | 609.1534 | Luteolin-3′, 7-di-O-glucoside | C27H30O16 | −0.6 | 563.2207, 471.0513, 447.2244, 430.9771, 285.0479, 267.0310, 112.9840 | |
| 59 | 7.23 | 595.2007 | Neoeriocitrin (Eriodictyol-7-O-neohesperidoside) | C27H32O15 | 1.1 | 577.1651, 449.1341, 287.1189 | |
| 61 | 7.32 | 595.1644 | Poncirin (Isosakuranetin-7-O-neohesperidoside) | C28H34O14 | 1.6 | 449.1119, 433.1142, 431.1005, 287.0566, 147.0629, 85.0291 | |
| 65 | 7.42 | 625.1824 | 623.1544 | Narcissin (Isorhamnetin-3-O-rutinoside) | C28H32O16 | −4.2 | 607.0872, 505.21542, 479.1170, 463.1479, 317.0589, 147.0355, 85.0486 |
| Flavonoid monoglycosides | |||||||
| 35 | 4.85 | 419.1354 | 417.1500 | Juglalin (Kaempferol-3-O-arabinoside) | C20H18O10 | 8.6 | 387.1057, 354.9223, 343.2181, 285.0213 |
| 39 | 5.22 | 433.1359 | Reynoutrin (Quercetin-3-O-xyloside) | C20H18O11 | 0.1 | 301.1242, 283.0096 | |
| 40 | 5.24 | 477.1637 | Isorhamnetin-3-O-glucoside | C22H22O12 | −5.8 | 431.1520,429.1815,401.1725, 315.0915, 285.0234, 227.0368 | |
| 42 | 5.70 | 465.1712 | Hyperoside (Quercetin-3-O-galactoside) | C21H20O12 | 0 | 345.0162, 375.0731, 303.0500, 285.0687, 247.1283, 229.0664, 153.0509 | |
| 43 | 5.87 | 481.1687 | Gossypin (Gossypetin-8-O-glucoside) | C21H20O13 | −0.1 | 391.0742, 361.1997, 319.1388, 169.1997 | |
| 44 | 6.02 | 447.1446 | 445.1336 | Baicalin (Baicalein-7-O-glucuronide) | C21H18O11 | 0.9 | 427.1506, 325.1046, 293.0912, 269.1125, 175.0739, 161.0405, 149.0441, 113.1216, 101.0233 |
| 50 | 6.50 | 417.1720 | Daidzin (Daidzein-7-O-glucoside) | C21H20O9 | 2.1 | 199.2364, 255.1957 | |
| 51 | 6.56 | 449.1086 | 447.0901 | Isoorientin (Luteolin-6-C-glucoside) | C21H20O11 | −3.3 | 431.0888, 359.0845, 329.0694, 287.0613, 251.1983 |
| 53 | 6.69 | 465.1013 | 463.0841 | Spiraeoside (Quercetin-4′-O-glucoside) | C21H20O12 | 1.1 | 432.0887, 303.0407, 285.1937, 229.0478, 163.0349, 153.0134, 137.0204 |
| 54 | 6.83 | 433.1491 | 431.0963 | Vitexin (Apigenin-8-C-glucoside) | C21H20O10 | 0 | 415.1018, 397.0891, 343.2051, 337.0742, 313.0685, 297.0719, 283.0607, 271.0547, 121.0298 |
| 56 | 7.02 | 593.1286 | Tiliroside | C30H26O13 | 0.4 | 307.0884, 285.0191, 284.0302, 255.0293, 227.0339, 151.0142 | |
| 60 | 7.28 | 417.1721 | 415.1568 | Puerarin (Daidzein-8-C-glucoside) | C21H20O9 | 0.9 | 397.1623, 369.1387, 295.0405, 267.0535, 253.1208, 179.0535 |
| 62 | 7.33 | 447.1349 | Quercitrin (Quercetin-3-O-rhamnoside) | C21H20O11 | −1.4 | 429.2153, 401.1227, 301.2084, 271.0723, 242.9609, 163.0403, 151.0251 | |
| 63 | 7.35 | 449.1419 | Orientin (Luteolin-8-C-glucoside) | C21H20O11 | −0.9 | 359.1362, 329.0694, 287.1072 | |
| 70 | 7.90 | 433.1152 | Prunin (Naringenin-7-O-glucoside) | C21H22O10 | −5.4 | 415.2054, 313.011, 271.0582, 256.9158, 228.9442 | |
| 81 | 15.98 | 431.2272 | Afzelin (Kaempferol-3-O-rhamnoside) | C21H20O10 | 0.9 | 340.9432, 285.0671, 255.9679, 163.04681 151.9852, 133.0201 | |
| Flavonoid aglycone | |||||||
| 41 | 5.62 | 305.0639 | 303.0313 | Taxifolin | C15H12O7 | 0.9 | 287.0832, 149.0264, 127.0152 |
| 46 | 6.17 | 319.1014 | 317.0557 | Myricetin | C15H10O8 | −0.4 | 289.1131, 245.0399, 181.10976,153.0727, 139.04166, 111.04249 |
| 66 | 7.59 | 287.0536 | 285.1381 | Luteolin | C15H10O6 | 2.7 | 269.16568, 259.05075, 241.13669, 219.0949, 231.06117, 153.0167, 135.0855 |
| 69 | 7.74 | 285.1326 | 283.1028 | Acacetin (Linarigenin) | C16H12O5 | −1.3 | 270.0095, 267.1167, 255.0842, 242.0842, 213.0459 |
| 74 | 9.02 | 269.1132 | Formononetin | C16H12O4 | 5 | 254.15146, 206.1075 | |
| 75 | 9.74 | 303.0495 | Quercetin | C15H10O7 | −0.7 | 285.0459, 275.0452, 259.1176, 257.0400, 247.0503, 229.0478, 195.0265, 165.1292, 153.0968 | |
| 76 | 11.40 | 315.0496 | Isorhamnetin | C16H12O7 | 2.6 | 301.1315, 300.0077, 283.0141, 151.0951 | |
| 79 | 14.29 | 269.1017 | Apigenin | C15H10O5 | −0.3 | 254.0987, 225.0989, 223.0218, 151.0379, 117.0294, 107.0346 | |
| 80 | 15.11 | 289.1795 | Eriodictyol | C15H12O6 | 0.6 | 201.88115, 179.1848, 153.18488, 135.0888 | |
| 83 | 19.94 | 317.1166 | Rhamnetin | C16H12O7 | −2.5 | 302.1045, 289.0751, 149.0220, 121.0259 | |
| 84 | 20.20 | 301.1427 | Kaempferide | C16H12O6 | −2 | 286.1130, 272.1159, 201.0075, 153.980, 135.0079 | |
| Coumarin | |||||||
| 30 | 3.45 | 177.0744 | 174.9555 | Hymecromone | C10H8O3 | 4.3 | 121.0974, 149.0219, 105.0720, 103.0552, 93.0764, 91.0520, 77.0383 |
| 33 | 4.05 | 193.0855 | Scopoletin | C10H8O4 | 0.9 | 133.0619, 115.0577 | |
| 36 | 4.87 | 339.2013 | Aesculin (Esculin) | C15H16O9 | −0.9 | 295.1424, 271.0700, 203.0817, 177.0179, 159.0881, 133.0132, 119.0471 | |
| 72 | 8.10 | 179.1077 | 177.0555 | Daphnetin (7,8-dihydroxycoumarin) | C9H6O4 | −0.2 | 133.1012, 107.0848, 105.0719, 77.0357 |
| Polyphenol | |||||||
| 27 | 2.76 | 229.1544 | Resveratrol | C14H12O3 | 0.6 | 214.0611, 187.0538, 185.0874, 170.0767, 145.0812 | |
| 45 | 6.05 | 579.1827 | Procyanidin B2 | C30H26O12 | 0.9 | Not fragmented | |
| 48 | 6.36 | 595.1667 | Antirrhinin (cyanidin-3-O-rutinoside) | C27H31O15 | −0.4 | 577.2179, 449.1097, 433.1143, 287.0569, 271.5852, 163.0382, 85.0287 | |
| 49 | 6.44 | 449.1088 | Marein (Okanin-4′-O-glucoside) | C21H22O11 | −0.8 | 431.1997, 401.1803, 287.0105, 153.0238, 135.0119 | |
| 58 | 7.15 | 611.1625 | 609.1437 | Tulipanin (Delphinidin 3-O-rutinoside) | C27H31O16 | −1.5 | 465.1013, 449.1120, 303.0505, 285.0316, 257.0490, 243.0799, 229.0521, 173.0621, 165.0679, 145.0493, 129.0269 |
| 64 | 7.38 | 449.1086 | Chrysanthemin (Cyanidin-3-O-glucoside) | C21H21O11 | −0.4 | 287.0522, 259.0772, 206.0878, 149.0908, 143.0493 | |
| 73 | 8.14 | 493.1316 | Primulin (Malvidin-3-galactoside) | C23H25O12 | 3.3 | 373.2349, 331.0705, 315.0639 | |
| 78 | 12.79 | 407.1350 | 405.1771 | Astringin | C20H22O9 | −1.1 | 243.0687, 225.0550, 201.0578, 179.0568, 161.0431, 159.0354 |
| 87 | 20.92 | 289.0696 | Epicatechin | C15H14O6 | −0.3 | 245.0605, 205.0152, 179.0347, 108.9038 | |
| Miscellaneous compounds | |||||||
| 3 | 1.17 | 168.0293 | Pyridoxine | C8H11NO3 | −0.4 | Not fragmented | |
| 4 | 1.18 | 133.0128 | Malic acid | C4H6O5 | 2 | 115.0044, 89.0230, 87.0105, 72.9940, 71.0132, 59.0171 | |
| 5 | 1.20 | 191.0570 | Citric acid | C6H8O7 | −2.2 | 173.0195, 130.9981, 129.0160, 111.0064, 87.0103, 85.0325 | |
| 8 | 1.23 | 135.0288 | γ-Terpinene | C10H16 | −0.5 | 117.0078 | |
| 10 | 1.32 | 122.0241 | Niacin (Nicotinic acid) | C6H5NO2 | 0.4 | 78.0322, 61.9938 | |
| 24 | 2.30 | 225.0666 | Carnosine | C9H14N4O3 | −7.4 | 130.0676, 113.0907, 89.0232 | |
| 26 | 2.60 | 220.1170 | 218.1039 | Pantothenate (Pantothenic acid) | C9H17NO5 | 1.5 | 202.1061, 184.0965, 124.0741, 116.0294, 98.0232, 90.0575, 85.0657 |
| 37 | 5.07 | 153.0536 | O-Anisic acid | C8H8O3 | 3.2 | 105.0371, 92.0273, 79.0541, 77.0380, 51.0239 | |
| 55 | 7.00 | 377.1448 | Riboflavin | C17H20N4O6 | 1.3 | 243.0886, 198.0633, 172.0824 | |
| 67 | 7.61 | 227.1278 | Hedione (Methyl dihydrojasmonate) | C13H22O3 | 0.9 | 209.1732, 195.0892, 167.1095, 153.1283 | |
| 68 | 7.66 | 225.1952 | Methyl Jasmonate | C13H20O3 | 3.3 | 194.9544, 165.0533, 143.1167, 55.0549 | |
| 71 | 8.04 | 411.1765 | γ-Tocotrienol | C28H42O2 | −0.5 | 409.8878, 242.1209 | |
| 77 | 11.85 | 169.1223 | Pyridoxamine | C8H12N2O2 | 0.1 | Not fragmented | |
| 82 | 19.90 | 149.0222 | Cinnamic acid | C9H8O2 | 4.4 | 131.0469, 105.0635, 103.0468, 75.0215, 53.0020 | |
| 85 | 20.26 | 277.2150 | γ-Linolenic acid | C18H30O2 | 6.7 | 259.2028, 233.2145, 231.2238, 205.1942, 59.0166 | |
| 86 | 20.80 | 281.1759 | Linoleic acid | C18H32O2 | −3.1 | 263.1773, 221.1796, 123.0819, 83.0433 | |
| 88 | 22.58 | 241.1969 | Anserine | C10H16N4O3 | −4.5 | 166.0745, 163.1030, 112.0812 | |
| Extract/Fractions | DPPH Assay IC50 µg/mL | ABTS Assay IC50 µg/mL | FRAP Assay EC50 µg/mL |
|---|---|---|---|
| BME | 254.3 ± 4.25 | 121.27 ± 4.8 | 384.7 ± 9.8 |
| BDMF | 289.0 ± 10.95 | 84.91 ± 2.46 | 255.4 ± 15.37 |
| BBF | 69.29 ± 1.77 | 15.59 ± 0.37 | 100.3 ± 4.26 |
| Trolox | 24.42 ± 0.87 | 22.56 ± 0.78 | 96.97 ± 2.73 |
| Extract/Fractions | AChE (IC50 µg/mL) |
|---|---|
| BME | 31.5 ± 1.6 |
| BDMF | >1000 |
| BBF | 16.4 ± 0.81 |
| Donepezil | 7.89 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, H.A.; Salama, A.M.; El-Toumy, S.A.; A. Salama, A.A.; Tadros, S.H.; El Gedaily, R.A. Novel Neuroprotective Potential of Bunchosia armeniaca (Cav.) DC against Lipopolysaccharide Induced Alzheimer’s Disease in Mice. Plants 2022, 11, 1792. https://doi.org/10.3390/plants11141792
Abbas HA, Salama AM, El-Toumy SA, A. Salama AA, Tadros SH, El Gedaily RA. Novel Neuroprotective Potential of Bunchosia armeniaca (Cav.) DC against Lipopolysaccharide Induced Alzheimer’s Disease in Mice. Plants. 2022; 11(14):1792. https://doi.org/10.3390/plants11141792
Chicago/Turabian StyleAbbas, Haidy A., Ahmed M. Salama, Sayed A. El-Toumy, Abeer A. A. Salama, Soad H. Tadros, and Rania A. El Gedaily. 2022. "Novel Neuroprotective Potential of Bunchosia armeniaca (Cav.) DC against Lipopolysaccharide Induced Alzheimer’s Disease in Mice" Plants 11, no. 14: 1792. https://doi.org/10.3390/plants11141792