Advances and Perspectives for Polyploidy Breeding in Orchids
Abstract
:1. Introduction
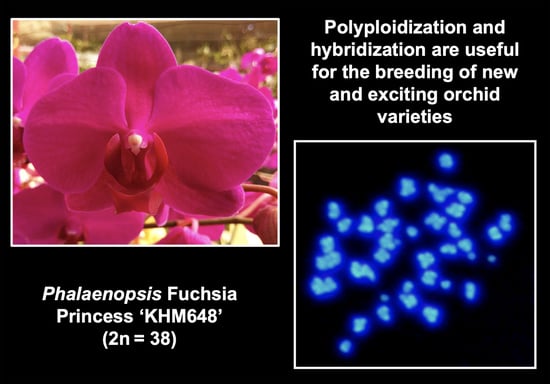
2. Polyploidy Breeding in Orchids
3. Chromosomal Pathways for Meiotic Polyploidization
4. Synapsis, Chromosome Segregation and Meiotic Non-Reduction
5. Cytokinesis, Heat Shock and Polyploidy
6. Recombination, Heat Shock and Polyploidy
7. Polyploidization and Recombination
8. Post-Polyploid Diploidization in Orchids
9. Induction of Polyploidy on Meiocytes
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolaños-Villegas, P.; Chin, S.-W.; Chen, F.-C. Meiotic Chromosome Behavior and Capsule Setting in Doritaenopsis Hybrids. J. Am. Soc. Hortic. Sci. 2008, 133, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.H.; Kao, Y.L.; Tang, C.Y.; Tsai, C.C.; Lin, T.Y. Estimating Nuclear DNA Content within 50 Species of the Genus Phalaenopsis Blume (Orchidaceae). Sci. Hortic. 2013, 161, 70–75. [Google Scholar] [CrossRef]
- Zeng, R.Z.; Zhu, J.; Xu, S.Y.; Du, G.H.; Guo, H.R.; Chen, J.; Zhang, Z.S.; Xie, L. Unreduced Male Gamete Formation in Cymbidium and Its Use for Developing Sexual Polyploid Cultivars. Front. Plant Sci. 2020, 11, 558. [Google Scholar] [CrossRef]
- Kamemoto, H.; D’Amore, T.; Kuenhle, A.R. Breeding Dendrobium Orchids in Hawaii, 1st ed.; University of Hawai’i Press: Honolulu, HI, USA, 1999. [Google Scholar]
- Lee, Y.-I.; Chung, M.C. Karyomorphological Observation on Some Paphiopedilum Hybrids. Acta Hortic. 2010, 878, 99–106. [Google Scholar] [CrossRef]
- Hu, C.J.; Lee, N.; Lee, Y.I. Meiotic Defects and Premature Tapetal Degeneration Are Involved in the Low Fertility of Oncidesa Gower Ramsey, an Important Cut-Flower Orchid. HortScience 2018, 53, 1283–1287. [Google Scholar] [CrossRef] [Green Version]
- Vo, T.C.; Lee, J.; Son, B.G.; Kim, C.K.; Kim, H.Y.; Lim, K.B. Phenotypic Correlation Analysis of Phalaenopsis Reciprocal F1 Hybrids Using SPSS and Principal Component Analysis (PCA). Acta Hortic. 2019, 1262, 213–218. [Google Scholar] [CrossRef]
- Silva, P.; Callegari-Jacques, S.; Bodanese-Zanettini, M. Induction and Identification of Polyploids in Cattleya intermedia Lindl. (Orchidaceae) by in vitro Techniques. Cienc. Rural 2000, 30, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, R.; Kamemoto, H. Meiotic Chromosome Behavior in Diploid and Polyploid Vanda Orchid Hybrids. Cytologia 1960, 25, 405–418. [Google Scholar] [CrossRef] [Green Version]
- Arends, J. Cytological Observations on Genome Homology in Eight Interspecific Hybrids of Phalaenopsis. Genetica 1970, 88–100. [Google Scholar] [CrossRef]
- Chin, S.W.; Cheng, T.C.; Chen, F.C. Orchid Improvement in Chinese Taipei. Acta Hortic. 2014, 1025, 189–194. [Google Scholar] [CrossRef]
- Yuan, S.-C.; Lekawatana, S.; Amore, T.D.; Chen, F.-C.; Chin, S.-W.; Vega, D.M.; Wang, Y.-T. The Global Orchid Market. In The Orchid Genome (Compendium of Plant Genomes); Chen, F.-C., Chin, S.-W., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–28. [Google Scholar] [CrossRef]
- Miguel, T.P.; Leonhardt, K.W. In Vitro Polyploid Induction of Orchids Using Oryzalin. Sci. Hortic. 2011, 130, 314–319. [Google Scholar] [CrossRef]
- Teoh, S.B.; Ong, E.C. Differential Meiotic Behaviour in Hybrids Clones of Aranda “Christine” (Orchidaceae). Euphytica 1983, 32, 799–806. [Google Scholar] [CrossRef]
- van de Peer, Y.; Mizrachi, E.; Marchal, K. The Evolutionary Significance of Polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar] [CrossRef]
- Yuan, S.-C.; Bolaños-Villegas, P.; Tsao, C.-Y.; Chen, F.-C. The Breeding of Phalaenopsis Hybrids. In The Orchid Genome. Compendium of Plant Genomes; Chen, F.-C., Chin, S.-W., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 29–40. [Google Scholar] [CrossRef]
- Pelé, A.; Rousseau-Gueutin, M.; Chèvre, A.-M. Speciation Success of Polyploid Plants Closely Relates to the Regulation of Meiotic Recombination. Front. Plant Sci. 2018, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Wall, W. Chromosomes: The Complex Code; Chapman and Hall Ltd.: London, UK, 1996. [Google Scholar]
- Pelé, A.; Falque, M.; Trotoux, G.; Eber, F.; Nègre, S.; Gilet, M.; Huteau, V.; Lodé, M.; Jousseaume, T.; Dechaumet, S.; et al. Amplifying Recombination Genome-Wide and Reshaping Crossover Landscapes in Brassicas. PLoS Genet. 2017, 13, e1006794. [Google Scholar] [CrossRef]
- Jones, K. Robertsonian Fusion and Centric Fission in Karyotype Evolution of Higher Plants. Bot. Rev. 1998, 64, 273–289. [Google Scholar] [CrossRef]
- Mandáková, T.; Lysak, M.A. Post-Polyploid Diploidization and Diversification through Dysploid Changes. Curr. Opin. Plant Biol. 2018, 42, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Song, Q.; Ye, W.; Chen, Z.J. Concerted Genomic and Epigenomic Changes Accompany Stabilization of Arabidopsis Allopolyploids. Nat. Ecol. Evol. 2021, 5, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wu, J.; Cai, X.; Liang, J.; Freeling, M.; Wang, X. Gene Retention, Fractionation and Subgenome Differences in Polyploid Plants. Nat. Plants 2018, 4, 258–268. [Google Scholar] [CrossRef]
- Mason, A.S.; Pires, J.C. Unreduced Gametes: Meiotic Mishap or Evolutionary Mechanism? Trends Genet. 2015, 31, 5–10. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, T.M.; Fu, Y.M.; Hsieh, R.M.; Chen, W.S. Studies on Somaclonal Variation in Phalaenopsis. Plant Cell Rep. 1998, 18, 7–13. [Google Scholar] [CrossRef]
- van de Peer, Y.; Ashman, T.L.; Soltis, P.S.; Soltis, D.E. Polyploidy: An Evolutionary and Ecological Force in Stressful Times. Plant Cell 2021, 33, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Hsiao, Y.Y.; Li, C.I.; Yeh, C.M.; Mitsuda, N.; Yang, H.X.; Chiu, C.C.; Chang, S.B.; Liu, Z.J.; Tsai, W.C. The Ancestral Duplicated DL/CRC Orthologs, PeDL1 and PeDL2, Function in Orchid Reproductive Organ Innovation. J. Exp. Bot. 2021, 72, 5442–5461. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liu, X.; Vanneste, K.; Proost, S.; Tsai, W.-C.; Liu, K.-W.; Chen, L.-J.; He, Y.; Xu, Q.; Bian, C.; et al. The Genome Sequence of the Orchid Phalaenopsis equestris. Nat. Genet. 2015, 47, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, K.; Schiestl, F.P. Are Tetraploids More Successful? Floral Signals, Reproductive Success and Floral Isolation in Mixed-Ploidy Populations of a Terrestrial Orchid. Ann. Bot. 2015, 115, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.K.; Yamamoto, M.; Mukai, Y. Delineation of Methylation and Histone Modification: The Epigenetic Regulatory Marks Show Slightly Altered Distribution with the Elevation in Ploidy Level in the Orchid Dendrobium nobile. Nucleus 2018, 61, 183–193. [Google Scholar] [CrossRef]
- Osabe, K.; Kawanabe, T.; Sasaki, T.; Ishikawa, R.; Okazaki, K.; Dennis, E.S.; Kazama, T.; Fujimoto, R. Multiple Mechanisms and Challenges for the Application of Allopolyploidy in Plants. Int. J. Mol. Sci. 2012, 13, 8696–8721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasio, F.; Prieto, P.; Pradillo, M.; Naranjo, T. Genomic and Meiotic Changes Accompanying Polyploidization. Plants 2022, 11, 125. [Google Scholar] [CrossRef]
- Chen, F.C.; Chin, S.W.; Huang, J.Z. Novel Orchid Breeding to Meet Future Market Demands. Acta Hortic. 2021, 1312, 1–7. [Google Scholar] [CrossRef]
- Bolaños-Villegas, P.; Chen, F.C. Cytological Identification of Chromosomal Rearrangements in Doritaenopsis and Phalaenopsis. J. Int. Coop. 2007, 2, 1–11. [Google Scholar] [CrossRef]
- Cui, L.; Sun, Y.; Xiao, K.; Wan, L.; Zhong, J.; Liu, Y.; Xie, Q.; Zhou, S. Analysis on the Abnormal Chromosomal Behaviour and the Partial Female Fertility of Allotriploid Lilium—‘Triumphator’ (LLO) Is Not Exceptional to the Hypothesis of Lily Interploid Hybridizations. Sci. Hortic. 2022, 293, 110746. [Google Scholar] [CrossRef]
- De Storme, N.; Copenhaver, G.P.; Geelen, D. Production of Diploid Male Gametes in Arabidopsis by Cold-Induced Destabilization of Postmeiotic Radial Microtubule Arrays. Plant Physiol. 2012, 160, 1808–1826. [Google Scholar] [CrossRef] [Green Version]
- Bretagnolle, F.; Thompson, J.D. Gametes with the Somatic Chromosome Number: Mechanisms of Their Formation and Role in the Evolution of Autopolyploid Plants. New Phytol. 1995, 129, 1–22. [Google Scholar] [CrossRef]
- Liu, B.; de Storme, N.; Geelen, D. Gibberellin Induces Diploid Pollen Formation by Interfering with Meiotic Cytokinesis. Plant Physiol. 2017, 173, 338–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, H.A.; Ellison, N.W.; Verry, I.M.; Williams, W.M. Asynapsis and Unreduced Gamete Formation in a Trifolium Interspecific Hybrid. BMC Plant Biol. 2022, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Zamariola, L.; Tiang, C.L.; de Storme, N.; Pawlowski, W.; Geelen, D. Chromosome Segregation in Plant Meiosis. Front. Plant Sci. 2014, 5, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Hamamura, Y.; Sofroni, K.; Böwer, F.; Stolze, S.C.; Nakagami, H.; Schnittger, A. SWITCH 1/DYAD Is a WINGS APART-LIKE Antagonist That Maintains Sister Chromatid Cohesion in Meiosis. Nat. Commun. 2019, 10, 1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolaños-Villegas, P. Chromosome Engineering in Tropical Cash Crops. Agronomy 2020, 10, 122. [Google Scholar] [CrossRef] [Green Version]
- Kuo, P.; da Ines, O.; Lambing, C. Rewiring Meiosis for Crop Improvement. Front. Plant Sci. 2021, 12, 1410. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Liu, Q.; Wang, C.; Qin, E.; Wu, Z.; Wang, M.; Eid Elesawi, I.; Chen, C.; Liu, H.; Qin, R.; et al. Heat Stress Interferes with Formation of Double-Strand Breaks and Homology Synapsis in Arabidopsis thaliana. bioRxiv 2020. [Google Scholar] [CrossRef]
- d’Erfurth, I.; Cromer, L.; Jolivet, S.; Girard, C.; Horlow, C.; Sun, Y.; To, J.P.C.; Berchowitz, L.E.; Copenhaver, G.P.; Mercier, R. The CYCLIN-A CYCA1;2/TAM Is Required for the Meiosis I to Meiosis II Transition and Cooperates with OSD1 for the Prophase to First Meiotic Division Transition. PLoS Genet. 2010, 6, e1000989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, X.; Ning, Y.; Eid Elesawi, I.; Yang, K.; Chen, C.; Wang, C.; Liu, B. Heat Stress Interferes with Chromosome Segregation and Cytokinesis during Male Meiosis in Arabidopsis thaliana. Plant Signal. Behav. 2020, 15, 1746985. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, A.; Lucas, M.-O.; Charpentier, C.; Sandmann, G.; Lloyd, A.; Jenczewski, E. Reducing MSH4 Copy Number Prevents Meiotic Crossovers between Non-Homologous Chromosomes in Brassica napus. Nat. Commun. 2019, 10, 2354. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Spielman, M.; Coles, J.P.; Li, Y.; Ghelani, S.; Bourdon, V.; Brown, R.; Lemmon, B.; Scott, R.; Dickinson, H. TETRASPORE Encodes a Kinesin Required for Male Meiotic Cytokinesis in Arabidopsis. Plant J. 2003, 34, 229–240. [Google Scholar] [CrossRef]
- Shahid, S. The Rules of Attachment: Rec8 Cohesin Connects Chromatin Architecture and Recombination Machinery in Meiosis. Plant Cell 2020, 32, 808–829. [Google Scholar] [CrossRef] [Green Version]
- Navarro, E.J.; Marshall, W.F.; Fung, J.C. Modeling Cell Biological Features of Meiotic Chromosome Pairing. bioRxiv 2022. [Google Scholar] [CrossRef]
- Bai, X.; Peirson, B.N.; Dong, F.; Xue, C.; Makaroff, C.A. Isolation and Characterization of SYN1, a RAD21-like Gene Essential for Meiosis in Arabidopsis. Plant Cell 1999, 11, 417–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crismani, W.; Girard, C.; Mercier, R. Tinkering with Meiosis. J. Exp. Bot. 2013, 64, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanday, I.; Skinner, D.; Yang, B.; Mercier, R.; Sundaresan, V. A Male-Expressed Rice Embryogenic Trigger Redirected for Asexual Propagation through Seeds. Nature 2019, 565, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.G.; Wu, F.H.; Yuan, Y.H.; Chen, Y.R.; Lin, C.S. High-Efficiency CRISPR/Cas-Based Editing of Phalaenopsis Orchid MADS Genes. Plant Biotechnol. J. 2020, 18, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.-T.; Yen, S.-H.; Yeh, J.-H.; Chen, W.-C.; Shih, M.-C. Orchidstra 2.0-A Transcriptomics Resource for the Orchid Family. Plant Cell Physiol. 2017, 58, e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulankova, P.; Akimcheva, S.; Fellner, N.; Riha, K. Identification of Arabidopsis Meiotic Cyclins Reveals Functional Diversification among Plant Cyclin Genes. PLoS Genet. 2013, 9, e1003508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wan, Y.; Meng, X.; Zhang, X.; Yao, M.; Miu, W.; Zhu, D.; Yuan, D.; Lu, K.; Li, J.; et al. Genome-Wide Identification and Analysis of Mkk and Mapk Gene Families in Brassica Species and Response to Stress in Brassica napus. Int. J. Mol. Sci. 2021, 22, 544. [Google Scholar] [CrossRef] [PubMed]
- France, M.G.; Enderle, J.; Röhrig, S.; Puchta, H.; Franklin, F.C.H.; Higgins, J.D. ZYP1 Is Required for Obligate Cross-over Formation and Cross-over Interference in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2021671118. [Google Scholar] [CrossRef] [PubMed]
- Capilla-Pérez, L.; Durand, S.; Hurel, A.; Lian, Q.; Chambon, A.; Taochy, C.; Solier, V.; Grelon, M.; Mercier, R.; Institut, B.; et al. The Synaptonemal Complex Imposes Crossover Interference and Heterochiasmy in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2023613118. [Google Scholar] [CrossRef]
- de Jaeger-Braet, J.; Krause, L.; Buchholz, A.; Schnittger, A. Heat Stress Reveals a Specialized Variant of the Pachytene Checkpoint in Meiosis of Arabidopsis thaliana. Plant Cell 2022, 34, 433–454. [Google Scholar] [CrossRef]
- Aoyama, M. Chromosome Research for Orchid Breeding. Acta Hortic. 2010, 878, 125–132. [Google Scholar] [CrossRef]
- Hsu, S.-T.; Chuang, H.-T.; Shen, T.-M. Breeding Barriers in Red Phalaenopsis Orchids. Acta Hortic. 2010, 878, 145–152. [Google Scholar] [CrossRef]
- Higgins, E.E.; Howell, E.C.; Armstrong, S.J.; Parkin, I.A.P. A Major Quantitative Trait Locus on Chromosome A9, BnaPh1, Controls Homoeologous Recombination in Brassica napus. New Phytol. 2021, 229, 3281–3293. [Google Scholar] [CrossRef] [PubMed]
- Enderle, J.; Dorn, A.; Beying, N.; Trapp, O.; Puchta, H. The Protease WSS1A, the Endonuclease MUS81, and the Phosphodiesterase TDP1 Are Involved in Independent Pathways of DNA-Protein Crosslink Repair in Plants. Plant Cell 2019, 31, 775–790. [Google Scholar] [CrossRef]
- Aklilu, B.B.; Soderquist, R.S.; Culligan, K.M. Genetic Analysis of the Replication Protein a Large Subunit Family in Arabidopsis Reveals Unique and Overlapping Roles in DNA Repair, Meiosis and DNA Replication. Nucleic Acids Res. 2014, 42, 3104–3118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teoh, S.B. Complement Fractionation in Natural Diploid Orchid Species. Theor. Appl. Genet. 1982, 61, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Edger, P.P.; Bird, K.A.; VanBuren, R.; Puzey, J.R. Tansley Insight: The Causes and Consequences of Subgenome Dominance in Hybrids and Recent Polyploids. New Phytol. 2018, 220, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Tsai, W.C.; Dievart, A.; Hsu, C.C.; Hsiao, Y.Y.; Chiou, S.Y.; Huang, H.; Chen, H.H. Post Genomics Era for Orchid Research. Bot. Stud. 2017, 58, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felix, L.P.; Guerra, M. Chromosome Analysis in Psygmorchis pusilla (L.) Dodson & Dressier: The Smallest Chromosome Number Known in Orchidaceae. Caryologia 1999, 52, 165–168. [Google Scholar] [CrossRef]
- Moraes, A.P.; Koehler, S.; Cabral, J.S.; Gomes, S.S.L.; Viccini, L.F.; Barros, F.; Felix, L.P.; Guerra, M.; Forni-Martins, E.R. Karyotype Diversity and Genome Size Variation in Neotropical Maxillariinae Orchids. Plant Biol. 2017, 19, 298–308. [Google Scholar] [CrossRef]
- Lee, Y.I.; Tseng, Y.F.; Lee, Y.C.; Chung, M.C. Chromosome Constitution and Nuclear DNA Content of Phalaenopsis Hybrids. Sci. Hortic. 2020, 262, 109089. [Google Scholar] [CrossRef]
- Grosso, V.; Farina, A.; Giorgi, D.; Nardi, L.; Diretto, G.; Lucretti, S. A High-Throughput Flow Cytometry System for Early Screening of in Vitro Made Polyploids in Dendrobium Hybrids. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 132, 57–70. [Google Scholar] [CrossRef]
- Dewitte, A.; Eeckhaut, T.; van Huylenbroeck, J.; van Bockstaele, E. Induction of 2n Pollen Formation in Begonia by Trifluralin and N2O Treatments. Euphytica 2010, 171, 283–293. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, J.; Sany, Y.; Zhao, Z.; Zhang, P.; Liu, M. Effects of Colchicine on Populus canescens Ectexine Structure and 2n Pollen Production. Front Plant Sci. 2020, 11, 295. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, P. Exposure to the Anthelmintic Dinitroaniline Oryzalin Causes Changes in Meiotic Prophase Morphology and Loss of Synaptonemal Complexes in the Nematode Caenorhabditis elegans. Exp. Results 2021, 2, e38. [Google Scholar] [CrossRef]
- Zhao, J.; Simmonds, D.H. Application of Trifluralin to Embryogenic Microspore Cultures to Generate Doubled Haploid Plants in Brassica napus. Physiol. Plant. 1995, 95, 304–309. [Google Scholar] [CrossRef]
- Hsu, S.C.; Cheng, T.C.; Bolaños-Villegas, P.; Chin, S.W.; Chen, F.C. Pollen Meiotic Behavior in Relation to Phalaenopsis Breeding. Acta Hortic. 2010, 878, 139–144. [Google Scholar] [CrossRef]


| Term | Description |
|---|---|
| Aneuploidy | Loss or gain of chromosomes relative to the normal chromosome complement [17] |
| Allopolyploid | Organism that combines two genomes or more deriving from related species [18] |
| Autotetraploid | A tetraploid plant formed directly from the merger of two unreduced gametes provided by the same diploid individual and formed via FDR [18] |
| Allotetraploid | A tetraploid formed from the merger of two unreduced gametes provided by different diploid plants and formed by FDR [18] |
| Amphihaploid | A haploid derived from unbalanced meiotic segregation in a hybrid [19] |
| Autopolyploid | Organism that combines two genomes or more deriving from the same species [18] |
| Bivalent | Pair of homologs physically linked at the end of metaphase I [18] |
| Centric fission | The break within the centromere of a single chromosome producing two whose raw ends can fuse after replication. Telomeric sequences may be added to the termini and two stable chromosomes are formed. Fission increases chromosome number and karyotype symmetry [20] |
| Chiasmata | The physical manifestation of reciprocal exchanges of DNA between non-sister chromatids (e.g., crossovers). Chiasmata maintain pairs of homologs linked at the end of metaphase I (as bivalents). At least one is required per bivalent to obtain well-balanced gametes and avoid aneuploidy [18] |
| Chromosomal Inversion | A structural change in chromosomes formed when two breaks are caused and the segment between them realigns and rejoins in the opposite orientation. To enable effective pairing in meiosis an inversion loop is formed. If a chiasma is formed within the loop the chromatids will form a dicentric bridge and an acentric fragment that is lost [18]. |
| Cytokinesis | Also known as cytoplasm division. It is the formation of a cell wall in plant cells at the telophase stage. Cellular organelles such as mitochondria are partitioned between the new daughter cells [18]. |
| Descending dysploidy | Evolutionary decrease in the base chromosome number (x). Also called polyploid drop and viewed as the mechanism that turns polyploids into functional diploids [21] |
| Dyad | A pair of cells instead of the usual four cells resulting from abnormal meiosis [4] |
| First Division Restitution (FDR) | A process in which a plant gamete is formed due to a defect in meiosis I. In these gametes there is neither recombination nor disjunction, thus parental heterozygosity is fully retained [19]. |
| Fractionation | The loss of one or the other copy of a newly duplicated gene [22] |
| Gene Dosage | Relative expression of a gene product [18]. Polyploids avoid imbalances in gene dosage by retaining duplicates and allowing them to eventually develop new functions [23]. |
| Genome Dominance | A process antagonistic to heterosis in which a subgenome gains relevance through dosage and functionalization and that may involve changes in chromatin marks and expression [23]. |
| Heterosis | The unevolved retention of genes or cis regulatory elements as homoeologous pairs, it is antagonistic to dominance [23]. It is linked to vigor in wide hybrids and allopolyploids. It may manifest as the acceleration of somatic growth including the enlargement of organs [23]. |
| Homoeologous | Non-homologous, often used to refer to meiotic pairing between non-homologous chromosomes. Homoeologous pairing reduces fertility [18]. |
| Homologous Recombination | Exchange of DNA sequences with the same linear arrangement of genes between a paternal and maternal chromosome copy, also called a homologous pair [24]. |
| Karyotype | Chromosome complement of an individual plant or species as described by number and morphology [4] |
| Micronuclei | Aberrant nuclei formed by the unequal distribution of chromosomes in daughter cells, often caused by unpaired chromosomes and common in tumor cell lines and meiotic cells of orchid hybrids [16,18]. |
| Multivalent | Association during meiosis of more than two chromosomes whose homologous regions are synapsed by pairs [4]. Multivalent formation is detrimental to meiotic chromosome segregation and reduces fertility [18]. |
| Ploidy | The basic chromosome set, often defined as ’x’. Thus, 2 x indicates that the organism has two basic sets of chromosomes. It is different from the number of chromosomes in zygotic cells defined as ’n´. For instance, bread wheat is a hexaploid in which 2n = 6x = 42, where x = 7 [18]. |
| Polyhaploid | Haploid individual resulting from polyploid parents [4] |
| Post-Polyploid Diploidization (PPD) | A process of evolutionary modifications that transform a polyploid genome into a quasi-diploid one. It is mediated by homoeologous recombination leading to structural chromosomal changes including reduction of chromosome number [21]. |
| Robertsonian Fusion | The fusion of two non-homologous chromosomes that gives rise to single chromosome with a single centromere [18,20]. As a result, the chromosome number is reduced, and the karyotype symmetry is increased [20]. |
| Second Division Restitution (SDR) | A process in which a plant gamete is formed due to a defect in meiosis II. The exclusive separation of recombined homologs causes the formation of partially homozygous unreduced gametes [17]. |
| Somaclonal Variation | Variation caused during tissue culture of orchids. Mutant plants can be distinguished by their morphological and physiological traits. It may be detected in the diploid karyotype of Phalaenopsis, but the specific chromosomal rearrangements are difficult to examine [25]. |
| Tetrad | The four haploid products of meiosis [4] |
| Tissue Culture | General term for the aseptic growth of tissues, calls and organs in vitro [4] |
| Univalents | Homologs that fail to pair and form chiasmata between them at metaphase I. Their behavior at anaphase I is unpredictable and may not engage in successful division [18]. |
| Unreduced Gamete | A gamete with the somatic chromosome complement [24]. |
| Whole Genome Duplication (WGD) | Events of whole genome doublings (tetraploidizations) or whole genome triplications that lead to the formation of autotetraploid, allotetraploid and hexaploid plants [22]. |
| Gene Name | Functional Features in the Literature and Databases 1,2,3 | Original Locus ID According to TAIR and NCBI 1,2 | Homologs of Interest in the Orchid Database Orchidstra 2.0 4 | Horticultural Use |
|---|---|---|---|---|
| ASY1NAPTIC1 (ASY1) | Encodes a protein with a HORMA-domain for recognition of chromatin states linked to DNA double-strand breaks, a peptidase domain, a Winged Helix (WH) DNA-binding domain and a SWIRM-domain for mediation of protein–protein interactions in the assembly of chromatin-protein complexes. ASY1 Participates in assembly of chiasmata and homologous chromosome pairing during meiosis. Mutants may show few chiasmata per nuclei. | At1g67370 | Phalaenopsis aphrodite transcript PATC148994 (E-Value: 1.32 × 10−142) | Heat-stress mediated disruption of synapsis and recombination. Formation of clonal gametes [44] |
| CYCA1;2/TARDY ASYNCHRONOUS MEIOSIS (TAM) | Encodes a N-terminal cyclin-like protein that may regulate cyclin dependent kinases (CDKs). Encodes a core cell cycle gene involved in meiosis II progression. Mutants develop dyads. | At1g77390 | Phalaenopsis modesta transcript PMTC011254 (E-Value: 7 × 10−94) | Formation of 2n gametes through SDR [45] |
| DYAD/SWITCH1 (SWI1) | Encodes a protein that features a catalytic phosphatidylinositol-specific phospholipase C X-domain that is probably involved in signal transduction. Protein intervenes in chromatid cohesion establishment, in chromosome structure during male and female meiosis, and in axial element formation. | At5g51330 | Phalaenopsis lueddemanniana transcript PLTC046384 (E-Value: 4 × 10−84) | Development of unreduced gametes by FDR [43] |
| ARABIDOPSIS THALIANA MAP KINASE 4 (MAPK4) | Encodes a protein with a conserved Mitogen-Activated Protein (MAP) kinase site. Required for male-specific meiotic cytokinesis. The mRNA is cell-to-cell mobile. | At4g01370 | Oncidium Gower Ramsey transcript OGTC022747 (E-Value: 6 × 10−13) | Formation of recombinant, SDR-type unreduced gametes [46] |
| ARABIDOPSIS THALIANA MAP KINASE KINASE 6 (MKK6) | Encodes a kinase protein with an ATP-binding site. Phosphorylates MAPK4. Required for male meiotic cytokinesis. | At5g56580 | Phalaenopsis aphrodite transcript PATC137812 (E-Value: 5 × 10−152) | Formation of recombinant, SDR-type unreduced gametes [46] |
| ARABIDOPSIS MUTS HOMOLOG 4 (MSH4) | Encodes a protein that features a core MutS, domain for DNA mismatch repair. It is involved in homologous chromosome segregation and meiotic mismatch repair during recombination. Mutants show low chiasmata formation. | At4g17380 | Phalaenopsis schilleriana transcript PSTC034674 (E-Value: 9 × 10−36) | Reduction of homoeologous recombination in polyploid hybrids, improved chromosome segregation [47] |
| MMS and UV Sensitive 81 (MUS81) | Encodes a protein that features a WH-like DNA-binding domain for branch migration and transcriptional repression, and an ERCC4 domain for cleaving branched structures generated during DNA repair, replication, and recombination. Mutants show few bivalents. | At4g30870 | Phalaenopsis lueddemanniana transcript PLTC011379 (E-Value: 2.82 × 10−69) | Reduction of homoeologous recombination in polyploid hybrids, improved chromosome segregation [47] |
| NPK1-ACTIVATING KINESIN 2/TETRASPORE (NACK2/TES) | Encodes a protein with a kinesin motor domain and a P-loop NTPase domain. It is required for cytokinesis in pollen. In mutants, all four microspore nuclei remain within the same cytoplasm after meiosis. | At3g43210 | Cymbidium ensifolium transcript CETC000340 (E-Value: 6 × 10−38) | Formation of recombinant, SDR-type unreduced gametes [48] |
| NPK1-RELATED PROTEIN KINASE 3 (NP3) | Encodes a protein with a Serine/Threonine kinase domain. Regulates microtubule organization. May regulate formation of the intersporal callose wall after male meiosis. Mutants may not complete meiotic cytokinesis. | At3g06030 | Phalaenopsis schilleriana transcript PSTC039874 (E-Value: 2 × 10−41) | Formation of recombinant, SDR-type unreduced gametes [46] |
| OMISSION OF SECOND DIVISION 1/GIGAS CELL 1 (OSD1/GIG1) | Encodes a protein from the Polychome protein. It may work as a negative regulator of the activity of the anaphase-promoting complex/cyclosome (APC/C) ubiquitin ligase. Mutants produce diploid gametes by skipping the second meiotic division. | At3g57860 | Phalaenopsis schilleriana transcript PSTC034707 (E-Value: 7 × 10−75) | Formation of 2n gametes through SDR [45] |
| PRECOCIOUS DISSOCIATION OF SISTERS 5B (PDS5B). | The respective protein contains an armadillo (ARM)-like fold, consisting of a multi-helical fold comprised of two curved layers of alpha helices that allow for proteins and nucleic acids. The Arabidopsis genome contains five orthologues that are required for proper chromosome segregation at anaphase I. | At1g77600 | Phalaenopsis schilleriana transcript PSTC040504 (E-Value: 9 × 10−30) | Stabilization of meiosis in hybrid polyploids [22] |
| RECOMBINATION 8/SYNAPTIC 1 (REC8/SYN1) | Encodes a RAD21-protein which may assemble as a hetero-tetramer that enables opening of SMC-kleisin rings. It is involved in chromosome condensation, pairing and segregation during meiosis. Responsible for cohesion between replicated sister chromatids. | At5g05490 | Phalaenopsis aphrodite transcript PATC192174 (E-Value: 9 × 10−44) | Heat-stress mediated disruption of synapsis and recombination. Formation of clonal gametes. Formation of unreduced gametes by FDR [44,49]. |
| REPLICATION PROTEIN A 1C (RPA1C) | Encodes a factor known as the Replication Protein A-70kDa-DNA-binding subunit. Contains an Oligonucleotide/Oligosaccharide Binding motif, or OB fold, a five-stranded beta-sheet coiled to bind single-stranded DNA. This protein regulates DNA unwinding during replication, recombination, and repair. Mutants show incomplete synapsis and meiotic chromosome fragmentation. | At5g45400 | Phalaenopsis bellina transcript PBTC027818 (E-Value: 2.70 × 10−128) | Reduction of homoeologous recombination in polyploid hybrids, improved chromosome segregation [47] |
| STRUCTURAL MAINTENANCE OF CHROMOSOMES 3/TITAN 7 (SMC3/TTN7) | May encode a member of the Structural Maintenance of Chromosomes (SMC) family of proteins. These proteins share a five-domain structure, with globular N- and C-terminal domains separated by a coiled-coil segment with a globular ‘‘hinge’’ domain. The N-terminal domain contains a ‘Walker A’ nucleotide-binding domain, while the C-terminal domain contains a ‘Walker B’ motif and an ATP-binding cassette (ABC). SMC3 localizes to the axial elements of pachytene chromosomes. Heterozygous mutants show reduced cohesion along the arms. | At2g27170 | Phalaenopsis equestris transcript PETC035815 (E-Value: 3.57 × 10−147) | Stabilization of meiosis in hybrid polyploids [22] |
| STRUCTURAL MAINTENANCE OF CHROMOSOMES 1/TITAN 8 (SMC1/TTN8) | May encode a SMC protein. Works together with SMC3 during the establishment of proper meiotic cohesion. | At3g54670 | Phalaenopsis bellina transcript PBTC023989 (E-Value: 3.25 × 10−122) | Stabilization of meiosis in hybrid polyploids [22] |
| STRUCTURAL MAINTENANCE OF CHROMOSOMES 6B/HYPERSENSITIVE TO MMS, IRRADIATION AND MMC; MIM (SMC6B/MIM) | May encode a protein part of the SMC5/6 complex. This complex promotes sister chromatid alignment and homologous recombination after DNA damage. Mutants produce unreduced gametes. | At5g61460 | Phalaenopsis equestris transcript PETC039012 (E-Value: 6.95 × 10−111) | Stabilization of meiosis in hybrid polyploids [22] |
| SYNAPTONEMAL COMPLEX PROTEIN 1A (ZYP1A) | May encode a myosin heavy chain-related protein. It may feature two coiled-coil domains and several sites for polar, basic, and acidic residues. It is involved in chromosome synapsis during meiosis I and localizes at the synaptonemal complex (SC), Mutants show improper or non-homologous synapsis. | At1g22260 | Phalaenopsis lueddemanniana transcript PLTC005471 (E-Value: 3.64 × 10−24) | Heat-stress mediated disruption of synapsis and recombination. Formation of unreduced gametes by FDR [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolaños-Villegas, P.; Chen, F.-C. Advances and Perspectives for Polyploidy Breeding in Orchids. Plants 2022, 11, 1421. https://doi.org/10.3390/plants11111421
Bolaños-Villegas P, Chen F-C. Advances and Perspectives for Polyploidy Breeding in Orchids. Plants. 2022; 11(11):1421. https://doi.org/10.3390/plants11111421
Chicago/Turabian StyleBolaños-Villegas, Pablo, and Fure-Chyi Chen. 2022. "Advances and Perspectives for Polyploidy Breeding in Orchids" Plants 11, no. 11: 1421. https://doi.org/10.3390/plants11111421