Narrow-Band 311 nm Ultraviolet-B Radiation Evokes Different Antioxidant Responses from Broad-Band Ultraviolet
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Photosystem II Is Limited by Long-Wavelength UV-B and Is a Potential Source of ROS
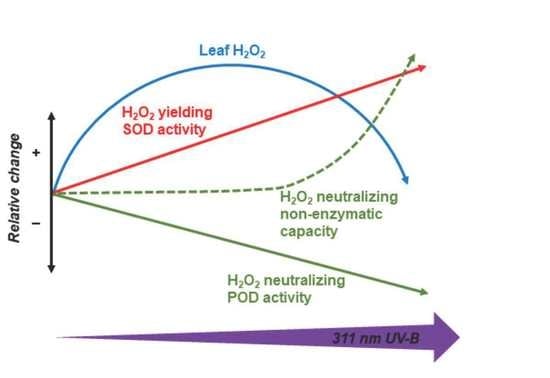
3.2. Long-Wavelength UV-B Tips the Balance between Enzymatic H2O2 Production and Scavenging
3.3. Non-Enzymatic Antioxidants Respond to Higher Fluxes of Long-Wavelength UV-B Than Enzymes
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Chlorophyll Fluorescence Measurements
4.3. Leaf Flavonoid Contents
4.4. Enzyme Activity Measurements
4.5. Non-Enzymatic Antioxidant Capacity Measurements
4.6. Leaf Hydrogen Peroxide Content Measurement
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant 2003, 119, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Majer, P.; Czégény, Gy.; Sándor, Gy.; Dix, P.J.; Hideg, É. Antioxidant defence in UV-irradiated tobacco leaves is centred on hydrogen-peroxide neutralization. Plant Physiol. Biochem. 2014, 82, 239–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czégény, Gy.; Le Martret, B.; Pávkovics, D.; Dix, P.J.; Hideg, É. Elevated ROS-scavenging enzymes contribute to acclimation to UV-B exposure in transplastomic tobacco plants, reducing the role of plastid peroxidases. J. Plant Physiol. 2016, 201, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rácz, A.; Hideg, É.; Czégény, Gy. Selective responses of class III plant peroxidase isoforms to environmentally relevant UV-B doses. J. Plant Physiol. 2018, 221, 101–106. [Google Scholar] [CrossRef]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Petrov, V.D.; Van Breusegem, F. Hydrogen peroxide—A central hub for information flow in plant cells. AoB Plants 2012, pls014. [Google Scholar] [CrossRef] [Green Version]
- Czégény, Gy.; Wu, M.; Dér, A.; Eriksson, L.A.; Strid, Å.; Hideg, É. Hydrogen peroxide contributes to the ultraviolet-B (280–315 nm) induced oxidative stress of plant leaves through multiple pathways. FEBS Lett. 2014, 588, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Mátai, A.; Nagy, D.; Hideg, É. UV-B strengthens antioxidant responses to drought in Nicotiana benthamiana leaves not only as supplementary irradiation but also as pretreatment. Plant Physiol. Biochem. 2019, 134, 9–19. [Google Scholar] [CrossRef]
- Flint, S.D.; Caldwell, M.M. A biological spectral weighting function for ozone depletion research with higher plants. Physiol. Plant. 2003, 117, 137–144. [Google Scholar] [CrossRef]
- Popova, V.; Ivanova, T.; Stoyanova, A.; Georgiev, V.; Hristeva, T.; Nikolova, V.; Docheva, M.; Nikolov, N.; Damyanova, S. Phytochemicals in leaves and extracts of the variety “Plovdiv 7” of Bulgarian oriental tobacco (Nicotiana tabacum L.). Trends Phytochem. Res. 2018, 2, 27–36. [Google Scholar]
- Rácz, A.; Czégény, Gy.; Csepregi, K.; Hideg, É. Ultraviolet-B acclimation is supported by functionally heterogeneous phenolic peroxidases. Sci. Rep. 2020, 10, 16303. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.A.; Cloix, C.; Jiang, G.H.; Kaiserli, E.; Herzyk, P.; Kliebenstein, D.J.; Jenkins, G.I. A UV-B-specific signaling component orchestrates plant UV protection. Proc. Natl. Acad. Sci. USA 2005, 102, 18225–18230. [Google Scholar] [CrossRef] [Green Version]
- Rizzini, L.; Favory, J.J.; Cloix, C.; Faggionato, D.; O’Hara, A.; Kaiserli, E.; Baumeister, R.; Schäfer, E.; Nagy, F.; Jenkins, G.I.; et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Christie, J.M.; Arvai, A.S.; Baxter, K.J.; Heilmann, M.; Pratt, A.J.; O’Hara, A.; Kelly, S.M.; Hothorn, M.; Smith, B.O.; Hitomi, K.; et al. Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science 2012, 335, 1492–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, G.I. Signal transduction in responses to UV-B radiation. Annu. Rev. Plant Biol. 2009, 60, 407–431. [Google Scholar] [CrossRef]
- Heijde, M.; Ulm, R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 2012, 17, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.I. Photomorphogenic responses to ultraviolet-B light. Plant Cell Environ. 2017, 40, 2544–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, R.; Ulm, R. How plants cope with UV-B: From perception to response. Curr. Opin. Plant Biol. 2017, 37, 42–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velanis, C.N.; Herzyk, P.; Jenkins, G.I. Regulation of transcription by the Arabidopsis UVR8 photoreceptor involves a specific histone modification. Plant Mol. Biol. 2016, 92, 425–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilian, J.; Whitehead, D.; Horak, J.; Wanke, D.; Weinl, S.; Batistic, O.; D’Angelo, C.; Bornberg-Bauer, E.; Kudla, J.; Harter, K. The AtGenExpress global stress expression data set: Protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 2007, 50, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Raggi, S.; Ferrarini, A.; Delledonne, M.; Dunand, C.; Ranocha, P.; De Lorenzo, G.; Cervone, F.; Ferrari, S. The Arabidopsis class III peroxidase AtPRX71 negatively regulates growth under physiological conditions and in response to cell wall damage. Plant Physiol. 2015, 169, 2513–2525. [Google Scholar] [CrossRef] [Green Version]
- Ślesak, I.; Libik, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Díaz-Ramos, L.A.; O’Hara, A.; Kanagarajan, S.; Farkas, D.; Strid, Å.; Jenkins, G.I. Difference in the action spectra for UVR8 monomerisation and HY5 transcript accumulation in Arabidopsis. Photochem. Photobiol. Sci. 2018, 17, 1108–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hara, A.; Headland, L.R.; Díaz-Ramos, L.A.; Morales, L.O.; Strid, Å.; Jenkins, G.I. Regulation of Arabidopsis gene expression by low fluence rate UV-B independently of UVR8 and stress signalling. Photochem. Photobiol. Sci. 2019, 18, 1675–1684. [Google Scholar] [CrossRef] [Green Version]
- Jansen, M.A.K.; Bilger, W.; Hideg, É.; Strid, Å.; UV4Plants Workshop Participants; Urban, O. Interactive effects of UV-B radiation in a complex environment. Plant Physiol. Biochem. 2019, 134, 1–8. [Google Scholar] [CrossRef]
- Ulm, R.; Nagy, F. Signalling and gene regulation in response to ultraviolet light. Curr. Opin. Plant Biol. 2005, 8, 477–482. [Google Scholar] [CrossRef]
- González Besteiro, M.A.; Bartels, S.; Albert, A.; Ulm, R. Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UV-B stress tolerance, independent of the UVR8 photoreceptor pathway. Plant J. 2011, 68, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soheila, A.-H.; Freda, J.C.; Jordan, B.T. Early signalling components in ultraviolet-B responses: Distinct roles for different reactive oxygen species and nitric oxide. FEBS Lett. 2001, 489, 237–242. [Google Scholar]
- Hideg, É.; Jansen, M.A.K.; Strid, Å. UV-B radiation ROS and stress; inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Ulm, R.; Jenkins, G.I. Q&A: How do plants sense and respond to UV-B radiation? BMC Biol. 2015, 13, 45. [Google Scholar]
- Czégény, Gy.; Mátai, A.; Hideg, É. UV-B effects on leaves—Oxidative stress and acclimation in controlled environments. Plant Sci. 2016, 248, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Czégény, Gy.; Kőrösi, L.; Strid, Å.; Hideg, É. Multiple roles for Vitamin B6 in plant acclimation to UV-B. Sci. Rep. 2019, 9, 125. [Google Scholar] [CrossRef]
- Aphalo, P.J.; Albert, A.; Björn, L.O.; McLeod, A.; Robson, T.M.; Rosenqvist, E. (Eds.) Beyond the Visible: A Handbook of Best Practice in Plant UV Photobiology; Department of Biosciences, Division of Plant Biology, University of Helsinki: Helsinki, Finland, 2012; p. 206. [Google Scholar]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the saturation pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Barta, C.; Kálai, T.; Hideg, K.; Vass, I.; Hideg, É. Differences in the ROS generating efficacy of various ultraviolet wavelengths in detached spinach leaves. Funct. Plant Biol. 2004, 31, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygen and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant. Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 2006, 1758, 994–1003. [Google Scholar] [CrossRef] [Green Version]
- Bienert, G.P.; Møller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef] [Green Version]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.; Moya, I. Dualex: A new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence. Appl. Opt. 2004, 43, 4488–4496. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Csepregi, K.; Hideg, É. A novel procedure to assess the non-enzymatic hydrogen-peroxide antioxidant capacity of metabolites with high UV absorption. Acta Biol. Hung. 2016, 67, 447–450. [Google Scholar] [CrossRef] [Green Version]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rácz, A.; Hideg, É. Narrow-Band 311 nm Ultraviolet-B Radiation Evokes Different Antioxidant Responses from Broad-Band Ultraviolet. Plants 2021, 10, 1570. https://doi.org/10.3390/plants10081570
Rácz A, Hideg É. Narrow-Band 311 nm Ultraviolet-B Radiation Evokes Different Antioxidant Responses from Broad-Band Ultraviolet. Plants. 2021; 10(8):1570. https://doi.org/10.3390/plants10081570
Chicago/Turabian StyleRácz, Arnold, and Éva Hideg. 2021. "Narrow-Band 311 nm Ultraviolet-B Radiation Evokes Different Antioxidant Responses from Broad-Band Ultraviolet" Plants 10, no. 8: 1570. https://doi.org/10.3390/plants10081570