Thymus serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of Thymus serpyllum Essential Oil
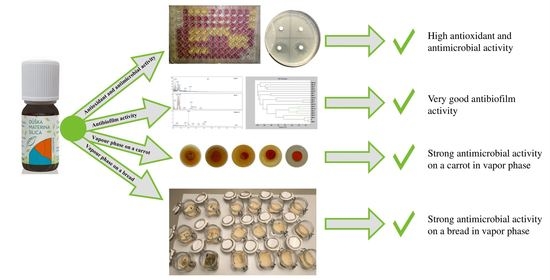
2.2. Antioxidant and Antimicrobial Activity, and Minimum Inhibitory Concentrations (MIC)
2.3. Analysis of Biofilm Development and Molecular Differences Using the MALDI-TOF MS Biotyper
2.4. Antimicrobial Analysis of Bread in Situ
2.5. In Situ Antimicrobial Analysis of Carrots
3. Discussion
4. Materials and Methods
4.1. Essential Oil
4.2. Tested Microorganisms
4.3. Chemical Characterization of Essential Oil Samples by Gas Chromatography/Mass Spectrometry (GC/MS) and Gas Chromatography (GC-FID)
4.4. Antioxidant Activity—DPPH Method
4.5. Antimicrobial Activity—Disc Diffusion Method
4.6. Minimum Inhibitory Concentrations (MIC)
4.7. Analysis of Differences in Biofilm Development with MALDI-TOF MS Biotyper
4.8. Antimicrobial Analysis of Bread In Situ
4.9. In Situ Antimicrobial Analysis on Carrots
4.10. Statistical Data Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of Ethnobotanical, Phytochemical, and Pharmacological Study of Thymus serpyllum L. Evid Based Complement Alternat. Med. 2015, 2015, 101978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, R.S.; Padalia, R.C.; Saikia, D.; Chauhan, A.; Krishna, V.; Sundaresan, V. Chemical composition and antimicrobial activity of the essential oils isolated from the herbage and aqueous distillates of two Thymus species. J. Essent. Oil. Bear. Plants 2014, 19, 936–943. [Google Scholar] [CrossRef]
- Ahmad, A.M.; Khokhar, I.; Ahmad, I.; Kashmiri, M.A.; Adnan, A.; Ahmad, M. Study of antimicrobial activity and composition by Gc/ms spectroscopic analysis of the essential oil of Thymus serphyllum. J. Food Saf. 2006, 5, 56–60. [Google Scholar]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; Griensven, L.J.L.D. Chemical Composition of Essential Oilsof Thymus and Mentha Speciesand Their Antifungal Activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Čabarkapa, I.; Čolović, R.; Đuragić, O.; Popović, S.; Kokić, B.; Milanov, D.; Pezo, L. Anti-biofilm activities of essential oils rich in carvacrol and thymol against Salmonella Enteritidis. Biofouling 2019, 35, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jin, P.; Gong, H.; Sun, Z.; Du, L.; Wang, D. Antibacterial and antibiofilm activities of thyme oil against foodborne multiple antibiotics-resistant Enterococcus faecalis. Poult. Sci. J. 2020, 99, 5127–5136. [Google Scholar] [CrossRef] [PubMed]
- Hong Wu, H.; Moser, C.; Wang, H.Z.; Høiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral. Sci. 2014, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial biofilm eradication agents: A current review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavian, D.; Nafchi, A.M.; Nouri, L.; Abedinia, A. Physicomechanical properties, release kinetics, and antimicrobial activity of activated low-density polyethylene and orientated polypropylene films by Thyme essential oil active component. J. Food Meas. Charact. 2021, 15, 883–891. [Google Scholar] [CrossRef]
- Jeican, I.I.; Tudoran, B.L.; Florea, A.; Flonta, M.; Trombitas, V.; Apostol, A.; Dumitru, M.; Aluaș, M.; Junie, L.M.; Albu, S. Chronic rhinosinusitis: MALDI-TOF mass spectrometry microbiological diagnosis and electron microscopy analysis; experience of the 2nd otorhinolaryngology clinic of Cluj-Napoca, Romania. J. Clin. Med. 2020, 9, 3973. [Google Scholar] [CrossRef] [PubMed]
- Naghdibadi, H.; Abdollahi, M.; Mehrafarin, A.; Ghorbanpour, M.; Tolyat, M.; Qaderi, A.; Yekta, M.G. An Overview on Two Valuable Natural and Bioactive Compounds, Thymol and Carvacrol, in Medicinal Plants. J. Med. Plants 2017, 16, 1–32. [Google Scholar]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, C.R.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crop. Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Baj, T.; Biernasiuk, A.; Wróbel, R.; Malm, A. Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata. Open Chem. 2020, 18, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Kovačević, Z.; Radinović, M.; Čabarkapa, I.; Kladar, N.; Božin, B. Natural agents against bovine mastitis pathogens. J. Antibiot. 2021, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Šojić, B.; Tomović, V.; Kocić-Tanackov, S.; Kovačević, D.B.; Putnik, P.; Mrkonjić, Ž.; Đurović, S.; Jokanović, M.; Ivić, M.; Škaljac, S.; et al. Supercritical extracts of wild thyme (Thymus serpyllum L.) by-product as natural antioxidants in ground pork patties. LWT Food. Sci. Technol. 2020, 130, 109661. [Google Scholar] [CrossRef]
- Tazabayeva, K.A.; Sylibaeva, B. Chemical composition of the essential oil and flavonoids of Thymus serpyllum L., growing on territory of the east Kazakhstan. Acta Pol. Pharm. 2018, 7, 1329–1337. [Google Scholar] [CrossRef]
- Pruteanu, A.; Popescu, C.; VLADUT, V.; GAGEANU, G. Biochemical analysis of some vegetal extracts obtained from indigenous spontaneous species of Thymus serpyllum L. Rom. Biotech. Lett. 2018, 23, 14013–14024. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019. [Google Scholar]
- European Medicines Agency Assessment Report on Thymus vulgaris L., Thymus zygis Loefl. ex. L., Aetheroleum. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-thymus-vulgaris-l-thymus-zygis-loefl-ex-l-aetheroleum_en.pdf (accessed on 15 March 2020).
- Kulisic, T.; Radonic, A.; Milos, M. Antioxidant properties of thyme (Thymus vulgaris L.) and wild thyme (Thymus serpyllum L.) essential oils. Ital. J. Food. Sci. 2005, 17, 315–324. Available online: https://www.researchgate.net/profile/Mladen-Milos/publication/287954724 (accessed on 20 April 2021).
- Hussain, A.I.; Anwar, F.; Chatha, S.A.S.; Latif, S.; Sherazi, S.T.H.; Ahmad, A.; Worthington, J.; Sarker, S.D. Chemical composition and bioactivity studies of the essential oils from two Thymus species from the Pakistani flora. LWT Food. Sci. Technol. 2013, 50, 185–192. [Google Scholar] [CrossRef]
- Goja, I.; Ulici, A.; Culea, M.; Munteanu, V.; Podea, P. The influence of geographic location and enzyme-assisted extraction on essential oils composition of Thymus serpyllum growing wild in Transylvania. Stud. Univ. Babes Bol. Chem. 2020, 65, 135–147. [Google Scholar] [CrossRef]
- Ouedrhiri, W.; Balouiri, M.; Bouhdid, S.; Moja, S.; Chahdi, O.F.; Taleb, M.; Greche, H. Mixture design of Origanum compactum, Origanum majorana and Thymus serpyllum essential oils: Optimization of their antibacterial effect. Ind. Crop. Prod. 2016, 80, 1–9. [Google Scholar] [CrossRef]
- Wesołowska, A.; Grzeszczuk, M.; Jadczak, D.; Nawrotek, P.; Struk, M. Comparison of the chemical composition and antimicrobial activity of Thymus serpyllum essential oils. Not. Bot. Horti Agrobo. 2015, 43, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Szabó, M.A.; Varga, G.Z.; Hohmann, J.; Schelz, Z.; Szegedi, E.; Amaral, L.; Molnár, J. Inhibition of Quorum-sensing Signals by Essential Oils. Phytother. Res. 2010, 24, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Camele, I.; Elshafie, H.S.; Caputo, L.; De Feo, V. Anti-quorum Sensing and Antimicrobial Effect of Mediterranean Plant Essential Oils Against Phytopathogenic Bacteria. Front. Microbiol. 2019, 10, 2619. [Google Scholar] [CrossRef] [PubMed]
- Božik, M.; Cejnar, P.; Šašková, M.; Nový, P.; Maršík, P.; Klouček, P. Stress response of Escherichia coli to essential oil components —Insights on low-molecular-weight proteins from MALDI-TOF. Sci. Rep. 2018, 8, 13042. [Google Scholar] [CrossRef] [PubMed]
- Kırmusaoğlu, S. Antimicrobials, Antibiotic, Resistance, Antibiofilm Strategies and Activity Methods, 1st ed.; IntechOpen: London, UK, 2019; pp. 99–102. [Google Scholar] [CrossRef]
- Stîngu, C.S.; Rodloff, A.C.; Jentsch, H.; Schaumann, R.; Eschrich, K. Rapid identification of oral anaerobic bacteria cultivated from subgingival biofilm by MALDI-TOF-MS. Oral. Microbiol. Immun. 2008, 23, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, A.M.; Labrie, J.; Goetz, C.; Dufour, S.; Jacques, M. Evaluation of MALDI-TOF mass spectrometry for the identification of bacteria growing as biofilms. J. Microbiol. Meth. 2018, 145, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P.A.D.F.; Menezes, R.P.; Penatti, M.P.A.; Moreira, T.A.; Pimenta, J.P.; Silva, N.B.S.; Röder, D.V.D.B. Rapid detection of biofilm-producing Candida species via MALDI-TOF mass spectrometry. J. Appl. Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Terentjeva, M.; Galovičová, L.; Ivanišová, E.; Štefániková, J.; Valková, V.; Borotová, P.; Kowalczewski, P.Ł.; Kunová, S.; Felšöciová, S.; et al. Biological activity and antibiofilm molecular profile of Citrus aurantium essential oil and its application in a food model. Molecules 2020, 25, 3956. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vukovic, N.L.; Štefániková, J.; Valková, V.; Borotová, P.; Žiarovská, J.; Terentjeva, M.; Felšöciová, S.; et al. Antioxidant, antimicrobial and antibiofilm activity of coriander (Coriandrum sativum L.) essential oil for its application in foods. Foods 2020, 9, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Střelková, T.; Nemes, B.; Kovács, A.; Novotný, D.; Božik, M.; Klouček, P. Inhibition of Fungal Strains Isolated from Cereal Grains via Vapor Phase of Essential Oils. Molecules 2021, 26, 1313. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Chen, J.; Zheng, X.; Liua, Q. Thyme oil to control Alternaria alternata in vitro and in vivo as fumigant and contact treatments. Food Control 2011, 22, 78–81. [Google Scholar] [CrossRef]
- Nedorostova, L.; Kloucek, P.; Kokoska, L.; Stolcova, M.; Pulkrabek, J. Antimicrobial properties of selected essential oils in vapour phase against foodborne bacteria. Food Control 2009, 20, 157–160. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial action of essential oil vapours and negative air ions against Pseudomonas fluorescens. Int. J. Food Microbiol. 2010, 143, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inouye, S.; Uchida, K.; Abe, S. Vapor activity of 72 essential oils against a Trichophyton mentagrophytes. J. Infect. Chemother. 2006, 12, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.A.; Cuffini, A.M.; Alonzo, V.; Carlone, N.A. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J. Appl. Microbiol. 2007, 102, 1544–1550. [Google Scholar] [CrossRef]
- Suhr, K.I.; Nielsen, P.V. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J. Appl. Microbiol. 2003, 94, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.; Bensoussan, M.; Gervais, P.; Dantigny, P. Inactivation of conidia of Penicillium chrysogenum, P. digitatum and P. italicum by ethanol solutions and vapours. Int. J. Food Microbiol. 2008, 122, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E.; Farrag, E.S. Antifungal activity of peppermint and sweet basil essential oils and their major aroma constituents on some plant pathogenic fungi from the vapor phase. Nahr. Food 2003, 47, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components By Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 469. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Bajčan, D.; Tomáš, J.; Uhlířová, G.; Árvay, J.; Trebichalský, P.; Stanovič, R.; Šimanský, V. Antioxidant potential of spinach, peas, and sweetcorn in relation to freezing period. Czech J. Food Sci. 2013, 31, 613–618. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.; Usman, J.; Kaleem, F.; Omair, M.; Khalid, A.; Iqbal, M. Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz. J. Infect. Dis. 2011, 15, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Talibi, I.; Askarne, L.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Aoumar, A.A.B. Antifungal activity of some Moroccan plants against Geotrichum candidum, the causal agent of postharvest citrus sour rot. Crop. Prot. 2013, 35, 41–46. [Google Scholar] [CrossRef]
- Aman, M.; Rai, V.R. Antifungal activity of fungicides and plant extracts against yellow sigatoka disease causing Mycosphaerella musicola. Curr. Res. Environ. Appl. Mycol. 2015, 5, 277–284. [Google Scholar] [CrossRef]






| Number | RI a | Compound b | % c |
|---|---|---|---|
| 1 | 858 | cis-hexen-1-ol | Tr |
| 2 | 926 | α-thujene | 0.5 |
| 3 | 938 | α-pinene | 1.2 |
| 4 | 948 | camphene | 1.1 |
| 5 | 976 | 1-octen-3-one | 0.8 |
| 6 | 980 | β-pinene | 0.3 |
| 7 | 992 | β-myrcene | 1.1 |
| 8 | 993 | octan-3-ol | Tr |
| 9 | 1004 | α-phellandrene | Tr |
| 10 | 1009 | δ-3-carene | Tr |
| 11 | 1016 | α-terpinene | 1.1 |
| 12 | 1026 | o-cymene | 15.4 |
| 13 | 1028 | α-limonene | 1.2 |
| 14 | 1033 | 1,8-cineole | 1.5 |
| 15 | 1060 | γ-terpinene | 8.1 |
| 16 | 1088 | α-terpinolene | Tr |
| 17 | 1098 | Linalool | 5.3 |
| 18 | 1148 | Camphor | 0.9 |
| 19 | 1170 | Borneol | 2.3 |
| 20 | 1178 | 4-terpineol | 1.8 |
| 21 | 1235 | Thymol methyl ether | Tr |
| 22 | 1241 | Carvone | Tr |
| 26 | 1245 | Carvacrol methyl ether | 0.5 |
| 27 | 1255 | Linalool acetate | 1.5 |
| 28 | 1256 | Geraniol | 10.7 |
| 29 | 1286 | Bornyl acetate | 0.6 |
| 30 | 1290 | Thymol | 18.8 |
| 31 | 1302 | Carvacrol | 17.4 |
| 32 | 1353 | α-cubebene | Tr |
| 33 | 1360 | Eugenol | Tr |
| 34 | 1379 | α-copaene | Tr |
| 35 | 1380 | Geranyl acetate | 4.4 |
| 36 | 1406 | Methyl eugenol | Tr |
| 37 | 1422 | cis-caryophyllene | 2.4 |
| 38 | 1456 | α-humulene | 1.0 |
| 39 | 1443 | Aromadendrene | Tr |
| 40 | 1507 | β-bisabolene | Tr |
| 41 | 1525 | δ-cadinene | Tr |
| 42 | 1583 | Caryophyllene oxide | Tr |
| Total | 99.9 |
| Microorganism | Inhibition Zone (mm) | Activity of EO | MIC 50 (µL/mL) | MIC 90 (µL/mL) | Activity of EO |
|---|---|---|---|---|---|
| Salmonella enteritidis | 15.67 ± 1.53 | *** | 0.39 | 0.78 | *** |
| Pseudomonas aeruginosa | 30.33 ± 0.58 | *** | 0.20 | 0.39 | *** |
| Yersinia enterocolitica | 6.33 ± 0.58 | * | 12.5 | 25.00 | * |
| Staphylococcus aureus | 8.33 ± 1.15 | * | 12.5 | 25.00 | * |
| Bacillus subtilis | 11.33 ± 1.53 | ** | 6.25 | 12.50 | ** |
| Enterococcus faecalis | 13.67 ± 1.53 | ** | 6.25 | 12.50 | ** |
| Candida albicans | 12.33 ± 1.53 | ** | 1.56 | 3.13 | *** |
| Candida krusei | 11.00 ± 1.00 | ** | 3.13 | 6.25 | *** |
| Candida tropicalis | 9.33 ± 0.58 | * | 12.5 | 25.00 | * |
| Candida glabrata | 11.00 ± 1.00 | ** | 3.13 | 6.25 | *** |
| Biofilm Stenotrophomonas maltophilia | 15.67 ± 0.58 | *** | 0.39 | 0.78 | *** |
| Biofilm Bacillus subtilis | 25.33 ± 0.58 | *** | 0.20 | 0.39 | *** |
| Bacterial Growth Inhibition (%) | ||||
|---|---|---|---|---|
| Concentration of EO | 62.5 µL/L | 125 µL/L | 250 µL/L | 500 µL/L |
| Microorganisms | ||||
| S. maltophilia | 11.13 ± 0.88 | −55.51 ± 2.07 | 44.05 ± 1.08 | 83.51 ± 0.54 |
| B. subtilis | −12.38 ± 1.22 | 30.50 ± 1.76 | 94.47 ± 2.73 | 84.21 ± 1.83 |
| Mycelial Growth Inhibition (%) | ||||
| Concentration of EO | 62.5 µL/L | 125 µL/L | 250 µL/L | 500 µL/L |
| Microorganisms | ||||
| P. citrinum | 84.93 ± 1.11 | 58.93 ± 1.97 | 73.27 ± 2.07 | 73.51 ± 1.33 |
| P. crustosum | 15.21 ± 0.43 | 42.63 ± 1.54 | 48.85 ± 2.63 | 54.15 ± 1.46 |
| P. expansum | 34.34 ± 2.41 | 84.93 ± 0.55 | 77.65 ± 1.89 | 66.26 ± 1.42 |
| Bacterial Growth Inhibition (%) | ||||
|---|---|---|---|---|
| Concentration of EO | 62.5 µL/L | 125 µL/L | 250 µL/L | 500 µL/L |
| Microorganisms | ||||
| S. maltophilia | 3.39 ± 0.09 | 25.72 ± 1.78 | 6.07 ± 1.32 | 83.77 ± 0.66 |
| B. subtilis | 42.92 ± 1.11 | 41.67 ± 2.09 | 47.15 ± 0.24 | 72.46 ± 2.07 |
| Mycelial Growth Inhibition (%) | ||||
| Concentration of EO | 62.5 µL/L | 125 µL/L | 250 µL/L | 500 µL/L |
| Microorganisms | ||||
| P. citrinum | −7.50 ± 0.88 | 63.27 ± 0.27 | 9.25 ± 1.75 | −7.50 ± 0.73 |
| P. crustosum | 44.20 ± 1.33 | 53.82 ± 0.86 | 23.24 ± 1.92 | 25.80 ± 1.01 |
| P. expansum | 0.85 ± 0.09 | 39.03 ± 1.45 | 8.75 ± 0.49 | 16.90 ± 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Terentjeva, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Kačániová, M. Thymus serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver. Plants 2021, 10, 1416. https://doi.org/10.3390/plants10071416
Galovičová L, Borotová P, Valková V, Vukovic NL, Vukic M, Terentjeva M, Štefániková J, Ďúranová H, Kowalczewski PŁ, Kačániová M. Thymus serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver. Plants. 2021; 10(7):1416. https://doi.org/10.3390/plants10071416
Chicago/Turabian StyleGalovičová, Lucia, Petra Borotová, Veronika Valková, Nenad L. Vukovic, Milena Vukic, Margarita Terentjeva, Jana Štefániková, Hana Ďúranová, Przemysław Łukasz Kowalczewski, and Miroslava Kačániová. 2021. "Thymus serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver" Plants 10, no. 7: 1416. https://doi.org/10.3390/plants10071416