Physico-Chemical, Nutritional, and Sensory Evaluation of Two New Commercial Tomato Hybrids and Their Parental Lines
Abstract
:1. Introduction
2. Results
2.1. Determination of Carbohydrates and Organic Acids
2.2. Determination of Phenolic Compounds
2.3. Fingerprinting of Volatile Components by ITEX/GC–MS Analysis
2.4. Correlations between the Pairs of the Main Chemical Compounds
2.5. Organoleptic Tastings—Taste and Flavor Results
2.6. The Relationships between the Chemical and Organoleptic Parameters
2.7. Heterosis
2.8. Relationships between the Analyzed Parameters
3. Discussion
3.1. Chemical Compounds Variations on Tomato Fruits
3.2. Sensory Analyses
3.3. Genetic Parameters and Their Utility in Tomatoes Breeding
3.4. General Considerations and Verification of Hypotheses—Judicious Choice of Cultivars used as Control, the Influence of Genotype on the Chemical Content of Useful Substances in Tomatoes and Conclusions of Organoleptic Tests
4. Materials and Methods
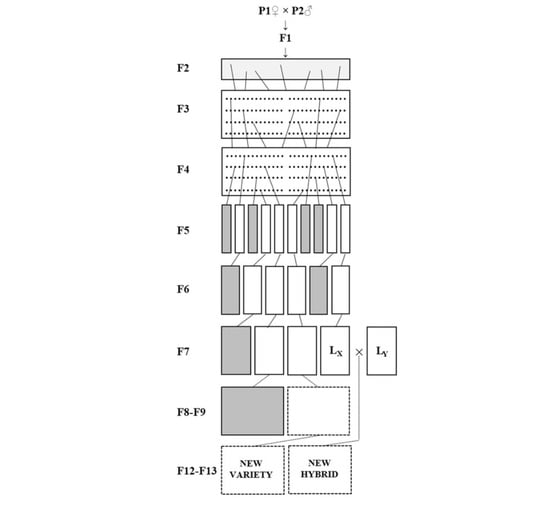
4.1. Cultivation Conditions and Plant Material
4.2. Chemicals and Equipment
4.3. Composition Traits
4.3.1. Determination of Carbohydrates
4.3.2. Determination of Organic Acids
4.3.3. Determination of Phenolic Compounds
4.4. Sensory Characteristics
4.4.1. Taste and Flavor Evaluation
4.4.2. Extraction of Volatile Compounds
4.5. Statistical and Multivariate Data Analysis
- -
- Absolute heterosis, using two ways:
- -
- comparing the trait (average value) of F1 hybrids with the average of the parents, using the formula:
- -
- comparing the trait (average value) of F1 hybrids with the average (trait value) of the best parent, using the formula:H = F1 − Pmax
- -
- Relative heterosis, or mid-parents heterosis (%) using the average values of F1 hybrids and genitors (parents), according to the formula:where F1 = mean value of the trait in F1; M.P. = mean value of the two parents, as (P1 + P2)/2.
- -
- Heterobeltiosis (BH), as a percentage, using the average of the F1 hybrids and the best parent (Pmax) for the analyzed trait, according to the formula:
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peralta, I.E.; Spooner, D.M. History, Origin and Early Cultivation of Tomato (Solanaceae). In Genetic Improvement of Solanaceous Crops Volume Tomato; Razdan, M.K., Matto, A.K., Eds.; Science Publishers: Plymouth, MA, USA, 2007; pp. 1–27. [Google Scholar]
- Bai, Y.; Lindhout, P. Domestication and Breeding of Tomatoes: What Have We Gained and What Can We Gain in the Future? 2007. Amm. Bot. 2007, 100, 1085–1094. [Google Scholar] [CrossRef]
- Long, J. Tomate y Jitomate en el Siglo XVI. Instituto de Investigaciones Históricas de la UNAM 2013. Available online: http://www.historicas.unam.mx/publicaciones/revistas/nahuatl/pdf/ecn25/463.pdf (accessed on 22 September 2015).
- Peralta, I.; Knapp, S.; Spooner, D. Nomenclature for wild and cultivated tomatoes. Tomato Genet. Coop. Rep. 2006, 56, 6–12. [Google Scholar]
- Bergougnoux, V. The history of tomato: From domestication to biopharming. Plant Biotechnol. 2013, 32, 170–189. [Google Scholar] [CrossRef]
- Rick, C.M.; Fobes, J.F. Allozyme variation in the cultivated tomato and closely related species. Bull. Torre Bot. Club 1975, 102, 376–384. [Google Scholar] [CrossRef]
- Blanca, J. Variation revealed by SNP genotyping and morphology provides insight into the origin of the tomato. PLoS ONE 2012, 7, e48198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanca, J.; Montero-Pau, J.; Sauvage, C.; Bauchet, G.; Illa, E.; Díez, M.J.; Francis, D.; Causse, M.; van der Knaap, E.; Cañizares, J. Genomic variation in tomato, from wild ancestors to contemporary breeding accessions. BMC Genom. 2015, 16, 257. [Google Scholar] [CrossRef] [Green Version]
- Razifard, H.; Ramos, A.; Della Valle, A.L.; Bodary, C.; Goetz, E.; Manser, E.J.; Li, X.; Zhang, L.; Visa, S.; Tieman, D.; et al. Genomic evidence for complex domestication history of the cultivated tomato in Latin America. Mol. Biol. Evol. 2020, 37, 1118–1132. [Google Scholar] [CrossRef] [PubMed]
- Peralta, I.E.; Spooner, D.M.; Knapp, S. Taxonomy of wild tomatoes and their relatives (Solanum sect. Lycopersicoides, sect. Juglandifolia, sect. Lycopersicon; Solanaceae). Syst. Bot. Monogr. 2008, 84, 1–186. [Google Scholar]
- Ichihashi, Y.; Sinha, N.R. From genome to phenome and back in tomato. Curr. Opin. Plant Biol. 2014, 18, 9–15. [Google Scholar] [CrossRef]
- Monforte, A.J.; Diaz, A.; Caño-Delgado, A.; Van Der Knaap, E. The genetic basis of fruit morphology in horticultural crops: Lessons from tomato and melon. J. Exp. Bot. 2014, 65, 4625–4637. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, G.R.; Muños, S.; Anderson, C.; Sim, S.-C.; Michel, A.; Causse, M.; Gardener, B.B.M.; Francis, D.; van der Knaap, E. Distribution of SUN, OVATE, LC, and FAS in the tomato germplasm and the relationship to fruit shape diversity. Plant Physiol. 2011, 156, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Paran, I.; Van Der Knaap, E. Genetic and molecular regulation of fruit and plant domestication traits in tomato and pepper. J. Exp. Bot. 2007, 58, 3841–3852. [Google Scholar] [CrossRef] [Green Version]
- García-Martínez, S.; Andreani, L.; Garcia-Gusano, M.; Geuna, F.; Ruiz, J.J. Evaluation of amplified fragment length polymorphism and simple sequence repeats for tomato germplasm fingerprinting: Utility for grouping closely related traditional cultivars. Genome 2006, 49, 648–656. [Google Scholar] [CrossRef]
- Sato, S.; Tabata, S.; Hirakawa, H.; Asamizu, E.; Shirasawa, K.; Isobe, S.; Kaneko, T.; Nakamura, Y.; Shibata, D.; Aoki, K.; et al. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635. [Google Scholar]
- Elbadrawy, E.; Sello, A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem. 2016, 9, S1010–S1018. [Google Scholar] [CrossRef] [Green Version]
- Abdullahi, I.I.; Abdullahi, N.; Abdu, A.M.; Ibrahim, A.S. Proximate, Mineral and Vitamin Analysis of Fresh and Canned Tomato. Biosci. Biotechnol. Res. Asia 2016, 13, 1163–1169. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; Romero-Gonzalez, R.; Gonzalez-Fernandes, M.J.; Guil-Guerrero, J.L. Phytochemical composition and in vitro anti-tumour activities of selected tomato varieties. J. Sci. Food Agric. 2017, 97, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef]
- Campestrini, L.H.; Melo, P.S.; Peres, L.E.P.; Calhelha, R.C.; Ferreira, I.C.; De Alencar, S.M. A new variety of purple tomato as a rich source of bioactive carotenoids and its potential health benefits. Heliyon 2019, 5, e02831. [Google Scholar] [CrossRef]
- Vats, S.; Bansal, R.; Rana, N.; Kumawat, S.; Bhatt, V.; Jadhav, P.; Kale, V.; Sathe, A.; Sonah, H.; Jugdaohsingh, R.; et al. Unexplored nutritive potential of tomato to combat global malnutrition. Crit. Rev. Food Sci. Nutr. 2020, 60, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.A.; Kader, A.A.; Albright-Holton, M. Intercultivar variation in composition of locular and pericarp portions of fresh market tomatoes. J. Am. Soc. Hortic. Sci. 1977, 102, 689–692. [Google Scholar]
- Malundo, M.M.; Shewfelt, R.L.; Scott, J.W. Flavor quality of fresh tomato (Lycopersicon esculentum Mill.) as affected by sugar and acid levels. Postharvest Biol. Technol. 1995, 6, 103–110. [Google Scholar] [CrossRef]
- Preedy, V.R.; Watson, R.R. Tomatoes and Tomato Products: Nutritional, Medicinal and Therapeutic Properties; Science Publishers: Enfield, NH, USA, 2008. [Google Scholar]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2020, 343, 128396. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-apoptotic effects of carotenoids in neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef]
- Helyes, L.; Lugasi, A.; Daood, H.G.; Pék, Z. The simultaneous effect of water supply and genotype on yield quantity, antioxidants content and composition of processing tomatoes. Notulae Botanicae Horti Agrobotanici 2014, 42, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Soto-Vaca, A.; Gutierrez, A.; Losso, J.N.; Xu, Z.; Finley, J.W. Evolution of phenolic compounds from color and flavor problems to health benefits. J. Agric. Food Chem. 2012, 60, 6658–6677. [Google Scholar] [CrossRef] [PubMed]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [Green Version]
- Krumbein, A.; Peters, P.; Brückner, B. Flavour compounds and a quantitative descriptive analysis of tomatoes (Lycopersicon esculentum Mill.) of different cultivars in short-term storage. Postharvest Biol. Technol. 2004, 32, 15–28. [Google Scholar] [CrossRef]
- Tikunov, Y.; Lommen, A.; Ric de Vos, C.H.; Verhoeven, H.A.; Bino, R.J.; Hall, R.D.; Bovy, A.G. A novel approach for nontargeted data analysis for metabolomics. Large-scale profiling of tomato fruit volatiles. Plant Physiol. 2005, 139, 1125–1137. [Google Scholar] [CrossRef] [Green Version]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martin-Belloso, O. Effect of minimal processing on bioactive compounds and color attributes of fresh-cut tomatoes. LWT 2008, 41, 217–226. [Google Scholar] [CrossRef]
- Rios, J.J.; Fernández-García, E.; Mínguez-Mosquera, I.; Pérez-Gálvez, A. Description of volatile compounds generated by the degradation of carotenoids in paprika, tomato and marigold oleoresins. Food Chem. 2008, 106, 1145–1153. [Google Scholar] [CrossRef]
- Lo Feudo, G.; Macchione, B.; Naccarato, A.; Sindona, G.; Tagarelli, A. The volatile fraction profiling of fresh tomatoes and triple concentrate tomato pastes as parameter for the determination of geographical origin. Food Res. Int. 2011, 44, 781–788. [Google Scholar] [CrossRef]
- Xu, N.; Gruber, M.; Westcott, N.; Soroka, J.; Parkin, I.; Hegedus, D. A Method for the Solvent Extraction of Low-boiling-point Plant Volatiles. Phytochem. Anal. 2005, 16, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2021, 10, 45. [Google Scholar] [CrossRef]
- Leyva, R.; Constán-Aguilar, C.; Blasco, B.; Sánchez-Rodríguez, E.; Romero, L.; Soriano, T.; Ruíz, J.M. Effects of climatic control on tomato yield and nutritional quality in Mediterranean screenhouse. J. Sci. Food Agric. 2014, 94, 63–70. [Google Scholar] [CrossRef]
- Carillo, P.; Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Dell’Aversana, E.; D’Amelia, L.; Colla, G.; Caruso, G.; De Pascale, S.; Rouphael, Y. Sensory and functional quality characterization of protected designation of origin ‘Piennolo del Vesuvio’ cherry tomato landraces from Campania-Italy. Food Chem. 2019, 292, 166–175. [Google Scholar] [CrossRef]
- Paolo, D.; Bianchi, G.; Morelli, C.F.; Speranza, G.; Campanelli, G.; Kidmose, U.; Lo Scalzo, R. Impact of drying techniques, seasonal variation and organic growing on flavor compounds profiles in two Italian tomato varieties. Food Chem. 2019, 298, 125062. [Google Scholar] [CrossRef] [Green Version]
- San Martín-Hernández, C.; Gómez-Merino, F.C.; Saucedo-Veloz, C.; Quintana-Obregón, E.A.; Muy-Rangel, M.D.; Trejo-Téllez, L.I. Nitrogen and potassium supplied by phenological stages affect the carotenoid and nutritive content of the tomato fruit. Notulae Botanicae Horti Agrobotanici 2021, 49, 12320. [Google Scholar] [CrossRef]
- Murariu, O.C.; Brezeanu, C.; Jităreanu, C.D.; Robu, T.; Irimia, L.M.; Trofin, A.E.; Popa, L.-D.; Stoleru, V.; Murariu, F.; Brezeanu, P.M. Functional quality of improved tomato genotypes grown in open field and in plastic tunnel under organic farming. Agriculture 2021, 11, 609. [Google Scholar] [CrossRef]
- Ladewig, P.; Trejo-Téllez, L.I.; Servín-Juárez, R.; Contreras-Oliva, A.; Gómez-Merino, F.C. Growth, yield and fruit quality of Mexican tomato landraces in response to salt stress. Notulae Botanicae Horti Agrobotanici 2021, 49, 12005. [Google Scholar] [CrossRef]
- Hernández-Suárez, M.; Rodrígues-Rodrígues, E.M.; Diaz Romero, C. Chemical composition of tomato (Lycopersicon esculentum) from Tenerife, the Canary Islands. Food Chem. 2008, 106, 1046–1056. [Google Scholar] [CrossRef]
- Fortis Hernandez, M.; Antonio-Ordoñez, E.; Preciado-Rangel, P.; Gallegos-Robles, M.A.; Vázquez-Vázquez, C.; Reyes-Gonzales, A.; Esparza-Rivera, J.R. Effect of substrates formulated with organic materials on yielding, commercial and phytochemical quality, and benefit-cost ratio of tomato (Solanum lycopersicum L.) produced under greenhouse conditions. Notulae Botanicae Horti Agrobotanici 2021, 49, 11999. [Google Scholar] [CrossRef]
- Agius, C.; von Tucher, S.; Poppenberger, B.; Rozhon, W. Quantification of sugars and organic acids in tomato fruits. MethodsX 2018, 5, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, P.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Sorrequieta, A.; Abriata, L.A.; Boggio, S.B.; Valle, E.M. Off-the-vine ripening of tomato fruit causes alteration in the primary metabolite composition. Metabolites 2013, 3, 967–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velterop, J.S.; Vos, F. A rapid and inexpensive microplate assay for the enzymatic determination of glucose, fructose, sucrose, L-malate and citrate in tomato (Lycopersicon esculentum) extracts and in orange juice. Phytochem. Anal. 2001, 12, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Missio, J.C.; Renau, R.M.; Artigas, F.C.; Cornejo, J.C. Sugar-and-acid profile of penjar tomatoes and its evolution during storage. Sci. Agric. 2015, 72, 314–321. [Google Scholar] [CrossRef]
- Porretta, S. Composizione del frutto del pomodoro. In Il Controllo Della Qualità dei Derivati del Pomodoro; Stazione Sperimentale per l’Industria Delle Conserve Alimentary: Parma, Italy, 1991; pp. 37–38. Available online: https://pomodoro.museidelcibo.it/wp-content/uploads/sites/5/2018/02/LIBRO-STAZIONE-SPERIMENTALE-CONSERVE.pdf (accessed on 14 October 2021).
- Ćetković, G.; Savatović, S.; Čanadanović-Brunet, J.; Djilas, S.; Vulić, J.; Mandić, A.; Četojević-Simin, D. Valorisation of phenolic composition, antioxidant and cell growth activities of tomato waste. Food Chem. 2012, 133, 938–945. [Google Scholar] [CrossRef]
- Valdez-Morales, M.; Espinosa-Alonso, L.G.; Espinoza-Torres, L.C.; Delgado-Vargas, F.; Medina-Godoy, S. Phenolic content and antioxidant and antimutagenic activities in tomato peel, seeds, and byproducts. J. Agric. Food Chem. 2014, 62, 5281–5289. [Google Scholar] [CrossRef]
- Barros, L.; Duenas, M.; Pinela, J.; Carvalho, A.M.; Santos, B.C.; Ferreira, I.C. Characterization and quantification of phenolic compounds in four tomato (Lycopersicon esculentum L.) farmers varieties in northeastern Portugal home gardens. Plants Foods Hum. Nutr. 2012, 67, 229–234. [Google Scholar] [CrossRef]
- Vallverdu’-Queralt, A.; Medina-Remo’n, A.; Casals-Ribes, I.; Andres-Lacueva, C.; Waterhouse, A.L.; Lamuela-Raventos, R.M. Effect of tomato industrial processing on phenolic profile and hydrophilic antioxidant capacity. LWT Food Sci. Technol. 2012, 47, 154–160. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Ayala-Zavala, J.F.; Dhua, R.S. Genotypic variation in tomatoes affecting processing and antioxidant properties. Crit. Rev. Food Sci. Nutr. 2015, 55, 1819–1835. [Google Scholar] [CrossRef] [PubMed]
- Del Río, D.; Rodríguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.B.; Bai, J.H.; Yu, Z.F. Difference in volatile profile between pericarp tissue and locular gel in tomato fruit. J. Integr. Agric. 2016, 15, 2911–2920. [Google Scholar] [CrossRef]
- Canoles, M.; Soto, M.; Beaudry, R. Hydroperoxide lyase activity necessary for normal aroma volatile biosynthesis of tomato fruit, impacting sensory perception and preference. Hortscience 2005, 40, 1130–1131. [Google Scholar] [CrossRef]
- Mathieu, S.; Cin, V.D.; Fei, Z.J.; Li, H.; Bliss, P.; Taylor, M.G.; Klee, H.J.; Tieman, D.M. Flavour compounds in tomato fruits: Identification of loci and potential pathways affecting volatile composition. J. Exp. Bot. 2009, 60, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Rambla, J.L.; Tikunov, Y.M.; Monforte, A.J.; Bovy, A.G.; Granell, A. The expanded tomato fruit volatile landscape. J. Exp. Bot. 2014, 65, 4613–4623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tieman, D.M.; Zeigler, M.; Schmelz, E.A.; Taylor, M.G.; Bliss, P.; Kirst, M.; Klee, H.J. Identification of loci affecting flavour volatile emissions in tomato fruits. J. Exp. Bot. 2006, 57, 887–896. [Google Scholar] [CrossRef] [Green Version]
- Selahle, M.K.; Sivakumar, D.; Soundy, P. Effect of photo-selective nettings on post-harvest quality and bioactive compounds in selected tomato cultivars. J. Sci. Food Agric. 2014, 94, 2187–2195. [Google Scholar] [CrossRef]
- Iijima, Y.; Iwasaki, Y.; Otagiri, Y.; Tsugawa, H.; Sato, T.; Otomo, H.; Sekine, Y.; Obata, A. Flavor characteristics of the juices from fresh market tomatoes differentiated from those from processing tomatoes by combined analysis of volatile profiles with sensory evaluation. Biosci. Biotechnol. Biochem. 2016, 80, 2401–2411. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.; Singh, G.K.; Patel, D.K. A Review on Pharmacological and Analytical Aspects of Naringenin. Chin. J. Integr. Med. 2014, 24, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.J.; Bozonnet, S.; Mullen, W.; Jenkins, G.I.; Lean, M.E.; Crozier, A. Occurrence of flavonols in tomatoes and tomato-based products. J. Agric. Food Chem. 2000, 48, 2663–2669. [Google Scholar] [CrossRef] [PubMed]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, E.A.; Scott, J.W.; Einstein, M.A.; Malundo, T.M.M.; Carr, B.T.; Shewfelt, R.L. Relationship between sensory and instrumental analysis for tomato flavour. J. Am. Soc. Hortic. Sci. 1998, 123, 906–915. [Google Scholar] [CrossRef] [Green Version]
- Bryun, J.W.; Garretsen, F.; Kooistra, E. Variation in taste and chemical composition of the tomato (Lycopersicon esculentum). Euphytica 1971, 20, 214–227. [Google Scholar] [CrossRef]
- Mikkelsen, R.L. Tomato Flavor and Plant Nutrition: A Brief Review. Better Crop. 2005, 89, 14–15. [Google Scholar]
- Baldwin, E.A.; Scott, J.W.; Shewfelt, R.L. Quality of ripened mutant and transgenic tomato cultigens. Proc. Tomato Qual. Wkshp. Proc. No. 1995, 503, 47–57. [Google Scholar]
- Maul, F. Harvest Maturity and Postharvest Temperature Management Can Compromise Flavor Potential of Fresh-Market Tomato (Lycopersicon esculentum Mill). Ph.D. Thesis, Horticulture Department, University of Florida, Gainesville, FL, USA, 1999. [Google Scholar]
- Maul, F.; Sargent, S.A.; Balaban, M.O.; Baldwin, E.A.; Huber, D.J.; Sims, C.A. Aroma volatile profiles from ripe tomatoes are influenced by phyiological maturity at harvest: An application of electronic nose technology. J. Amer. Soc. Hort. Sci. 1998, 123, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Kader, A.A.; Morris, L.L.; Stevens, M.A.; Albright-Holton, M. Composition and flavor quality of fresh market tomatoes as influenced by some postharvest handling procedures. J. Amer. Soc. Hort. Sci. 1978, 103, 6–13. [Google Scholar]
- Dan, C.; Șerban, C.; Sestraş, A.F.; Militaru, M.; Morariu, P.; Sestraş, R.E. Consumer Perception Concerning Apple Fruit Quality, Depending on Cultivars and Hedonic Scale of Evaluation—A Case Study. Not. Sci. Biol. 2015, 7, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Racskó, J.; Miller, D.D.; Duarte, E.E.; Szabó, Z.; Soltész, M.; Nyéki, J.; Szukics, J. Is consumer preference for apple driven only by fruit quality? Acta Hort. 2009, 831, 331–338. [Google Scholar] [CrossRef]
- Acquaah, G. Principles of Plant Genetics and Breeding, 1st ed.; Blackwell Publishing: Hoboken, NJ, USA, 2007. [Google Scholar]
- Liu, Z.; Jiang, J.; Ren, A.; Xu, X.; Zhang, H.; Zhao, T.; Jiang, X.; Sun, Y.; Li, J.; Yang, H. Heterosis and Combining Ability Analysis of Fruit Yield, Early Maturity, and Quality in Tomato. Agronomy 2021, 11, 807. [Google Scholar] [CrossRef]
- Yordanov, M. Heterosis in the tomato. In Heterosis, Reappraisal of Theory and Practice; Frankel, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 189–219. [Google Scholar]
- Kumar, R.; Srivastava, K.; Kumar, V.; Saroj, S.K.; Sharma, S.K.; Singh, R.K. Heterosis analysis in tomato (Solanum lycopersicum L.) for Lycopene, TSS, titrable acidity and Ascorbic acid. Electron. J. Plant Breed. 2019, 10, 1547. [Google Scholar] [CrossRef]
- Semel, Y.; Nissenbaum, Y.; Menda, N.; Zinder, M.; Issman, N.; Krieger, U.; Pleban, T.; Lippman, Z.; Gur, A.; Zamir, D. Overdominant QTL for yield and fitness in tomato. Proc. Natl. Acad. Sci. USA 2006, 103, 12981–12986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaeppler, S. Heterosis: Many Genes, Many Mechanisms—End the Search for an Undiscovered Unifying Theory. ISRN Bot. 2012, 2012, 682824. [Google Scholar] [CrossRef] [Green Version]
- Avdikos, I.D.; Nteve, G.-M.; Apostolopoulou, A.; Tagiakas, R.; Mylonas, I.; Xynias, I.N.; Papathanasiou, F.; Kalaitzis, P.; Mavromatis, A.G. Analysis of Re-Heterosis for Yield and Fruit Quality in Restructured Hybrids, Generated from Crossings among Tomato Recombinant Lines. Agronomy 2021, 11, 822. [Google Scholar] [CrossRef]
- Singh, B.D.; Singh, A.K. Marker-Assisted Plant Breeding: Principles and Practices; Springer: New Delhi, India, 2015; pp. 7–8. [Google Scholar]
- Pherobase. Available online: www.pherobase.com (accessed on 10 June 2021).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 11 June 2021).
- Mather, K.; Jinks, J.L. Biometrical Genetics, 2nd ed.; Chapman and Hall: London, UK, 1971; pp. 65–68. [Google Scholar]
- Baciu, A.D.; Pamfil, D.; Sestras, A.F.; Mihalte, L.; Sestras, R.E. Heterosis Studies for Response to Aphis fabae Attack in Calendula. Bulletin of the University of Agricultural Sciences & Veterinary Medicine Cluj-Napoca. Horticulture 2012, 69, 40–47. [Google Scholar]
- Solieman, T.; El-Gabry, M.; Abido, A. Heterosis, potence ratio and correlation of some important characters in tomato (Solanum lycopersicum L.). Sci. Hortic. 2013, 150, 25–30. [Google Scholar] [CrossRef]












| No. | Tomato Genotype | Glucose | Fructose | Glucose + Fructose | Malic Acid | Citric Acid | Malic Acid + Citric Acid | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Signif. | Mean ± SD | Signif. | Mean ± SD | Signif. | Mean ± SD | Signif. | Mean ± SD | Signif. | Mean ± SD | Signif. | ||
| 1 | Precos F1 | 7.17 ± 0.55 | b | 9.91 ± 0.60 | b | 17.08 ± 1.15 | b | 0.61 ± 0.10 | e | 2.40 ± 0.04 | f | 3.01 ± 0.14 | c |
| 2 | AS 30 ♀ (F7) | 7.57 ± 0.42 | b | 10.09 ± 0.37 | b | 17.66 ± 0.80 | b | 1.53 ± 0.06 | b | 2.91 ± 0.03 | d | 4.44 ± 0.09 | b |
| 3 | AS 31 ♂ (F7) | 7.59 ± 0.46 | b | 11.17 ± 0.76 | b | 18.76 ± 1.22 | b | 1.35 ± 0.04 | c | 3.00 ± 0.05 | c | 4.35 ± 0.01 | b |
| 4 | AS 300 F1 | 5.75 ± 0.54 | c | 7.63 ± 0.85 | c | 13.38 ± 1.39 | c | 0.94 ± 0.07 | d | 1.73 ± 0.07 | g | 2.83 ± 0.21 | c |
| 5 | Addalyn F1 | 12.15 ± 0.57 | a | 14.67± 0.62 | a | 26.82 ± 1.18 | a | 1.73 ± 0.09 | a | 3.33 ± 0.08 | b | 5.06 ± 0.17 | a |
| 6 | AS 09 ♀ (F7) | 11.38 ± 0.85 | a | 15.71 ± 1.02 | a | 27.09 ± 1.87 | a | 1.53 ± 0.05 | b | 2.72 ± 0.01 | e | 4.24 ± 0.04 | b |
| 7 | AS 10 ♂ (F7) | 12.52 ± 0.78 | a | 15.30 ± 0.54 | a | 27.82 ± 0.23 | a | 1.52 ± 0.09 | b | 3.39 ± 0.03 | a | 4.90 ± 0.12 | a |
| 8 | AS 400 F1 | 11.37 ± 0.81 | a | 15.11 ± 0.93 | a | 26.47 ± 1.74 | a | 1.63 ± 0.05 | ab | 2.70 ± 0.03 | e | 4.33 ± 0.09 | b |
| No. | Tomato Genotype | Caffeic-Glucoside I | Caffeic-Glucoside II | 3-Caffeoyl Quinic Acid | 5-Caffeoyl Quinic Acid | Caffeic Acid | Ferulic-Glucoside | Q-Tri Glucoside | Q-Rutinoside | Naringenin | Ferulic Acid | Total Phenolic Compounds | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Signif. | Mean ± SD | Signif. | Mean ± SD | Signif. | Mean ± SD | Signif. | Mean ± SD | Signif. | Mean ± SD | Signif. | Mean ± SD | Signif. | Mean ± SD | Signif. | Mean ± SD | Signif. | Mean ± SD | Signif. | Mean ± SD | Signif. | ||
| 1 | Precos F1 | 6.8 ± 0.5 | d | 4.0 ± 0.2 | e | 6.5 ± 0.5 | cde | 6.9 ± 0.5 | de | 3.0 ± 0.4 | c | 6.1 ± 0.2 | a | 3.6 ± 0.4 | de | 2.6 ± 0.4 | f | 1.6 ± 0.1 | d | 6.8 ± 0.5 | c | 47.8 ± 2.6 | de |
| 2 | AS 30 ♀ (F7) | 2.5 ± 0.3 | f | 3.3 ± 0.6 | e | 5.7 ± 0.1 | de | 6.4 ± 0.5 | e | 2.6 ± 0.3 | c | 2.4 ± 0.4 | e | 3.1 ± 0.4 | e | 3.4 ± 0.6 | e | 2.5 ± 0.6 | c | 6.7 ± 0.4 | c | 38.4 ± 4.1 | f |
| 3 | AS 31 ♂ (F7) | 3.2 ± 0.6 | f | 3.5 ± 0.2 | e | 5.4 ± 0.6 | e | 7.2 ± 0.4 | de | 3.1 ± 0.4 | c | 3.8 ± 0.6 | cd | 3.8 ± 0.4 | cde | 4.1 ± 0.8 | d | 2.1 ± 0.3 | cd | 6.8 ± 0.8 | c | 42.8 ± 3.5 | ef |
| 4 | AS 300 F1 | 4.7 ± 0.9 | e | 5.0 ± 0.6 | d | 6.8 ± 0.6 | cd | 7.9 ± 0.6 | d | 4.2 ± 0.5 | b | 4.2 ± 0.5 | bc | 3.9 ± 0.6 | cd | 3.7 ± 0.6 | e | 2.2 ± 0.6 | cd | 8.0 ± 0.6 | b | 50.3 ± 6.1 | de |
| 5 | Addalyn F1 | 5.6 ± 0.6 | e | 7.4 ± 0.6 | c | 7.8 ± 0.6 | c | 12.0 ± 0.8 | c | 4.4 ± 0.3 | b | 3.2 ± 0.4 | d | 3.7 ± 0.3 | cde | 3.6 ± 0.3 | e | 2.3 ± 0.1 | c | 3.6 ± 0.4 | e | 53.4 ± 3.0 | d |
| 6 | AS 09 ♀ (F7) | 16.8 ± 0.6 | a | 10.2 ± 0.7 | a | 12.5 ± 0.6 | b | 16.2 ± 0.9 | ab | 6.0 ± 0.4 | a | 5.5 ± 0.4 | a | 7.8 ± 0.1 | a | 6.5 ± 0.4 | c | 4.8 ± 0.4 | a | 10.1 ± 0.7 | a | 96.3 ± 5.3 | a |
| 7 | AS 10 ♂ (F7) | 9.8 ± 0.8 | b | 9.0 ± 0.5 | b | 14.3 ± 0.6 | a | 17.1 ± 0.6 | a | 5.8 ± 0.4 | a | 4.6 ± 0.4 | b | 6.8 ± 0.5 | b | 10.2 ± 0.4 | a | 5.1 ± 0.5 | a | 6.0 ± 0.8 | c | 88.4 ± 4.5 | b |
| 8 | AS 400 F1 | 8.5 ± 0.5 | c | 7.4 ± 0.1 | bc | 13.3 ± 0.9 | ab | 15.0 ± 0.5 | b | 5.5 ± 0.5 | a | 3.8 ± 0.6 | cd | 4.5 ± 0.5 | c | 7.6 ± 0.4 | b | 3.6 ± 0.4 | b | 4.6 ± 0.4 | d | 73.4 ± 3.8 | c |
| r. | Traits (Chemical Component) | Coefficient of Correlation (‘r’ Calculated Value between Pair of Traits; 1–17 Traits)/‘p’ Value | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | ||
| 1 | Glucose | 0.000 | 0.000 | 0.037 | 0.044 | 0.022 | 0.119 | 0.685 | 0.379 | 0.167 | 0.708 | 0.886 | 0.340 | 0.132 | 0.554 | 0.845 | 0.783 | |
| 2 | Fructose | 0.973 | 0.000 | 0.040 | 0.060 | 0.032 | 0.063 | 0.832 | 0.328 | 0.206 | 0.725 | 0.916 | 0.240 | 0.219 | 0.547 | 0.736 | 0.784 | |
| 3 | Glucose + fructose | 0.992 | 0.994 | 0.037 | 0.050 | 0.025 | 0.083 | 0.761 | 0.348 | 0.183 | 0.715 | 0.992 | 0.280 | 0.172 | 0.548 | 0.784 | 0.783 | |
| 4 | Malic acid | 0.738 | 0.729 | 0.738 | 0.050 | 0.002 | 0.640 | 0.428 | 0.753 | 0.393 | 0.277 | 0.359 | 0.331 | 0.782 | 0.915 | 0.823 | 0.945 | |
| 5 | Citric acid | 0.719 | 0.687 | 0.707 | 0.707 | 0.001 | 0.852 | 0.836 | 0.832 | 0.734 | 0.116 | 0.855 | 0.360 | 0.770 | 0.348 | 0.743 | 0.241 | |
| 6 | Malic acid + citric acid | 0.781 | 0.751 | 0.770 | 0.908 | 0.937 | 0.767 | 0.591 | 0.977 | 0.531 | 0.142 | 0.745 | 0.331 | 0.774 | 0.642 | 0.917 | 0.551 | |
| 7 | Caffeic-glucoside I | 0.597 | 0.681 | 0.647 | 0.197 | 0.080 | 0.125 | 0.567 | 0.506 | 0.082 | 0.721 | 0.705 | 0.195 | 0.262 | 0.953 | 0.381 | 0.430 | |
| 8 | Caffeic-glucoside II | 0.172 | 0.090 | 0.129 | 0.327 | 0.088 | 0.225 | 0.240 | 0.230 | 0.032 | 0.203 | 0.422 | 0.188 | 0.714 | 0.491 | 0.993 | 0.188 | |
| 9 | 3-Caffeoyl quinic | 0.361 | 0.398 | 0.384 | 0.133 | −0.090 | 0.012 | 0.277 | −0.479 | 0.836 | 0.112 | 0.224 | 0.512 | 0.635 | 0.646 | 0.855 | 0.958 | |
| 10 | 5-Caffeoyl quinic | 0.541 | 0.501 | 0.523 | 0.352 | 0.144 | 0.262 | 0.649 | 0.749 | 0.088 | 0.511 | 0.436 | 0.086 | 0.215 | 0.779 | 0.841 | 0.392 | |
| 11 | Caffeic | −0.158 | −0.149 | −0.154 | −0.439 | −0.600 | −0.568 | 0.151 | −0.504 | 0.604 | −0.274 | 0.575 | 0.045 | 0.823 | 0.390 | 0.724 | 0.403 | |
| 12 | Ferulic-glucoside | −0.061 | 0.045 | −0.004 | 0.376 | −0.078 | 0.138 | −0.160 | −0.332 | 0.484 | −0.323 | 0.235 | 0.555 | 0.151 | 0.417 | 0.888 | 0.565 | |
| 13 | Q-tri glucoside | −0.390 | −0.470 | −0.436 | −0.396 | −0.375 | −0.397 | −0.512 | −0.518 | 0.274 | −0.643 | 0.719 | 0.247 | 0.605 | 0.388 | 0.919 | 0.792 | |
| 14 | Q-rutinoside | −0.580 | −0.489 | −0.535 | −0.118 | −0.124 | −0.122 | −0.451 | −0.155 | −0.200 | −0.493 | −0.095 | 0.557 | 0.217 | 0.439 | 0.274 | 0.648 | |
| 15 | Naringenin | −0.248 | −0.252 | −0.252 | 0.045 | −0.384 | −0.196 | 0.025 | 0.287 | −0.194 | −0.119 | 0.354 | 0.335 | 0.355 | 0.321 | 0.668 | 0.011 | |
| 16 | Ferulic | 0.083 | 0.143 | 0.116 | −0.095 | 0.139 | 0.045 | 0.360 | 0.003 | 0.077 | 0.085 | 0.149 | 0.060 | 0.043 | 0.441 | 0.181 | 0.403 | |
| 17 | Total phenolic compounds | −0.117 | −0.116 | −0.117 | 0.029 | −0.469 | −0.250 | 0.326 | 0.519 | 0.022 | 0.352 | 0.345 | 0.241 | 0.112 | 0.193 | 0.829 | 0.345 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felföldi, Z.; Ranga, F.; Socaci, S.A.; Farcas, A.; Plazas, M.; Sestras, A.F.; Vodnar, D.C.; Prohens, J.; Sestras, R.E. Physico-Chemical, Nutritional, and Sensory Evaluation of Two New Commercial Tomato Hybrids and Their Parental Lines. Plants 2021, 10, 2480. https://doi.org/10.3390/plants10112480
Felföldi Z, Ranga F, Socaci SA, Farcas A, Plazas M, Sestras AF, Vodnar DC, Prohens J, Sestras RE. Physico-Chemical, Nutritional, and Sensory Evaluation of Two New Commercial Tomato Hybrids and Their Parental Lines. Plants. 2021; 10(11):2480. https://doi.org/10.3390/plants10112480
Chicago/Turabian StyleFelföldi, Zoltán, Floricuta Ranga, Sonia Ancuta Socaci, Anca Farcas, Mariola Plazas, Adriana F. Sestras, Dan Cristian Vodnar, Jaime Prohens, and Radu E. Sestras. 2021. "Physico-Chemical, Nutritional, and Sensory Evaluation of Two New Commercial Tomato Hybrids and Their Parental Lines" Plants 10, no. 11: 2480. https://doi.org/10.3390/plants10112480