Characterization of 15-cis-ζ-Carotene Isomerase Z-ISO in Cultivated and Wild Tomato Species Differing in Ripe Fruit Pigmentation
Abstract
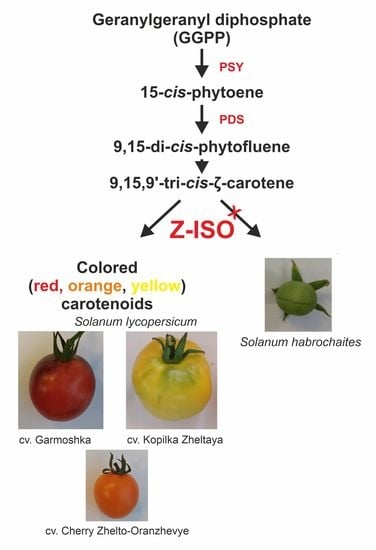
:1. Introduction
2. Results
2.1. Identification and Structural and Phylogenetic Characterization of Z-ISO Homologs in Tomato Cultivars and Wild Species
2.2. In Silico Analysis of S. lycopersicum Z-ISO Expression
2.3. Analysis of Z-ISO Promoters
2.4. Z-ISO Gene Expression in Tomato Cultivars and Wild Species
2.5. Carotenoid and Chloropohyll Content in the Fruit of Tomato Cultivars and Wild Species
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Identification and Structural Characterization of Tomato Z-ISO Genes
4.3. In Silico Analysis of mRNA Expression
4.4. RNA Extraction and Quantitative Real-Time PCR (RT-qPCR)
4.5. Promoter and 5′-UTR Identification and Analysis
4.6. Carotenoid and Chlorophyll Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From Plants to Food and Feed Industries. Methods Mol. Biol. 2018, 1852, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Park, W.J.; Lee, G.M.; Oh, C.S.; Yeam, I.; Won, D.C.; Kim, C.K.; Lee, J.M. Inferring the genetic determinants of fruit colors in tomato by carotenoid profiling. Molecules 2017, 22, 764. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Shao, Z.; Zhang, M.; Wang, Q. Regulation of carotenoid metabolism in tomato. Mol. Plant 2015, 8, 28–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Ren, M.; Peng, P.; Chun, Y.; Li, L.; Zhao, J.; Fang, J.; Peng, L.; Yan, J.; Chu, J.; et al. MIT1, encoding a 15-cis-ζ-carotene isomerase, regulates tiller number and stature in rice. J. Genet. Genom. 2021, 48, 88–91. [Google Scholar] [CrossRef]
- Beltrán, J.; Kloss, B.; Hosler, J.P.; Geng, J.; Liu, A.; Modi, A.; Dawson, J.H. Control of carotenoid biosynthesis through a heme-based cis-trans isomerase. Nat. Chem. Biol. 2015, 11, 598–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Li, F.; Wurtzel, E.T. Isolation and characterization of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants. Plant Physiol. 2010, 153, 66–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Yang, M.; Zhao, L.; Zhu, Z.; Liu, F.; Sun, H.; Sun, C.; Tan, L. High-tillering and dwarf 12 modulates photosynthesis and plant architecture by affecting carotenoid biosynthesis in rice. J. Exp. Bot. 2021, 72, 1212–1224. [Google Scholar] [CrossRef]
- Davison, P.A.; Hunter, C.N.; Horton, P. Overexpression of beta-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 2002, 418, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Murillo, C.; Wurtzel, E.T. Maize Y9 encodes a product essential for 15-cis zeta-carotene isomerization. Plant Physiol. 2007, 144, 1181–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.L.; Hsu, Y.T.; Kang, C.Y.; Lee, T.M. Nitric oxide down-regulation of carotenoid synthesis and PSII activity in relation to very high light-induced singlet oxygen production and oxidative stress in Chlamydomonas reinhardtii. Plant Cell Physiol. 2013, 54, 1296–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, D.S. Phenotypic and genetic studies of a new mutant of yellow endosperm in maize. J. Hered. 1975, 66, 127–130. [Google Scholar] [CrossRef]
- Janick-Buckner, D.; O’Neal, J.; Joyce, E.; Buckner, B. Genetic and biochemical analysis of the y9 gene of maize, a carotenoid biosynthetic gene. Maydica 2001, 46, 41–46. [Google Scholar]
- Sugiyama, K.; Takahashi, K.; Nakazawa, K.; Yamada, M.; Kato, S.; Shinomura, T.; Nagashima, Y.; Suzuki, H.; Ara, T.; Harada, J.; et al. Oxygenic Phototrophs Need ζ-Carotene Isomerase (Z-ISO) for Carotene Synthesis: Functional Analysis in Arthrospira and Euglena. Plant Cell Physiol. 2020, 61, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, Q.; Yan, J.; Sun, K.; Liang, Y.; Jia, M.; Meng, X.; Fang, S.; Wang, Y.; Jing, Y.; et al. ζ-Carotene Isomerase Suppresses Tillering in Rice through the Coordinated Biosynthesis of Strigolactone and Abscisic Acid. Mol. Plant 2020, 13, 1784–1801. [Google Scholar] [CrossRef]
- Cazzonelli, C.I.; Hou, X.; Alagoz, Y.; Rivers, J.; Dhami, N.; Lee, J.; Marri, S.; Pogson, B.J. A cis-carotene derived apocarotenoid regulates etioplast and chloroplast development. Elife 2020, 9, e45310. [Google Scholar] [CrossRef] [PubMed]
- Beltran Zambrano, J.A. Functional Characterization of the Plant 15-cis-Zeta-Carotene Isomerase Z-Iso. Ph.D. Thesis, City University of New York (CUNY), New York, NY, USA, 2015. [Google Scholar]
- Berry, H.M.; Rickett, D.V.; Baxter, C.J.; Enfissi, E.M.A.; Fraser, P.D. Carotenoid biosynthesis and sequestration in red chilli pepper fruit and its impact on colour intensity traits. J. Exp. Bot. 2019, 70, 2637–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, L.; Rodriguez-Concepcion, M. Manipulation of Plastidial Protein Quality Control Components as a New Strategy to Improve Carotenoid Contents in Tomato Fruit. Front. Plant Sci. 2019, 10, 1071. [Google Scholar] [CrossRef]
- Peralta, I.E.; Knapp, S.; Spooner, D.M. Nomenclature for wild and cultivated tomatoes. Rep. Tomato Genet. Coop. 2006, 56, 6–10. [Google Scholar]
- Peralta, I.E.; Spooner, D.M. History, origin and early cultivation of tomato (Solanaceae). Genet. Improv. Solanaceous Crop 2006, 2, 1–24. [Google Scholar]
- Sathasivam, R.; Yeo, H.J.; Park, C.H.; Choi, M.; Kwon, H.; Sim, J.E.; Park, S.U.; Kim, J.K. Molecular Characterization, Expression Analysis of Carotenoid, Xanthophyll, Apocarotenoid Pathway Genes, and Carotenoid and Xanthophyll Accumulation in Chelidonium majus L. Plants 2021, 10, 1753. [Google Scholar] [CrossRef] [PubMed]
- Galpaz, N.; Ronen, G.; Khalfa, Z.; Zamir, D.; Hirschberg, J. A chromoplast-specific carotenoid biosynthesis pathway is revealed by cloning of the tomato white-flower locus. Plant Cell 2006, 18, 1947–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peralta, I.E.; Spooner, D.M.; Knapp, S. Taxonomy of wild tomatoes and their relatives (Solanum sect. Lycopersicoides, sect. Juglandifolia, sect. Lycopersicon; Solanaceae). Syst. Bot. Monogr. 2008, 84, 1–186. [Google Scholar]
- Töro, I.; Petrutz, C.; Pacello, F.; D’Orazio, M.; Battistoni, A.; Djinovic-Carugo, K. Structural basis of heme binding in the Cu,Zn superoxide dismutase from Haemophilus ducreyi. J. Mol. Biol. 2009, 386, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.J.; Lado, J.; Alós, E.; Alquézar, B.; Dery, O.; Hirschberg, J.; Zacarías, L. A mutant allele of ζ-carotene isomerase (Z-ISO) is associated with the yellow pigmentation of the “Pinalate” sweet orange mutant and reveals new insights into its role in fruit carotenogenesis. BMC Plant Biol. 2019, 19, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koul, A.; Sharma, D.; Kaul, S.; Dhar, M.K. Identification and in silico characterization of cis-acting elements of genes involved in carotenoid biosynthesis in tomato. 3 Biotech 2019, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Coku, A.; Inoue, K.; Tian, L. Expression, subcellular localization and cis-regulatory structure of duplicated phytoene synthase genes in melon (Cucumis melo L.). Planta 2011, 234, 737–748. [Google Scholar] [CrossRef]
- Efremov, G.I.; Slugina, M.A.; Shchennikova, A.V.; Kochieva, E.Z. Differential Regulation of Phytoene Synthase PSY1 during Fruit Carotenogenesis in Cultivated and Wild Tomato Species (Solanum section Lycopersicon). Plants 2020, 9, 1169. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Nakano, T.; Shima, Y.; Ito, Y. A large-scale identification of direct targets of the tomato MADS box transcription factor Ripening Inhibitor reveals the regulation of fruit ripening. Plant Cell. 2013, 25, 371–386. [Google Scholar] [CrossRef] [Green Version]
- Slugina, M.A. Transcription Factor Ripening Inhibitor and Its Homologs in Regulation of Fleshy Fruit Ripening of Various Plant Species. Russ. J. Plant Physiol. 2021, 68, 783–799. [Google Scholar] [CrossRef]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.P.; Bouzayen, M. Ethylene Control of Fruit Ripening: Revisiting the Complex Network of Transcriptional Regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef] [Green Version]
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Kim, S.T.; Gupta, R. Ethylene: A Master Regulator of Salinity Stress Tolerance in Plants. Biomolecules 2020, 10, 959. [Google Scholar] [CrossRef]
- Osorio, C.E. The Role of Orange Gene in Carotenoid Accumulation: Manipulating Chromoplasts toward a Colored Future. Front. Plant Sci. 2019, 10, 1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilambi, H.V.; Manda, K.; Rai, A.; Charakana, C.; Bagri, J.; Sharma, R.; Sreelakshmi, Y. Green-fruited Solanum habrochaites lacks fruit-specific carotenogenesis due to metabolic and structural blocks. J. Exp. Bot. 2017, 68, 4803–4819. [Google Scholar] [CrossRef] [PubMed]
- Tanksley, S.D.; McCouch, S.R. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science 1997, 277, 1063–1066. [Google Scholar] [CrossRef] [Green Version]
- Grumet, R.; Fobes, J.F.; Herner, R.C. Ripening behavior of wild tomato species. Plant Physiol. 1981, 68, 1428–1432. [Google Scholar] [CrossRef]
- Chevalier, C.; Nafati, M.; Mathieu-Rivet, E.; Bourdon, M.; Frangne, N.; Cheniclet, C.; Renaudin, J.P.; Gvaudant, F.; Hernould, M. Elucidating the functional role of endoreduplication in tomato fruit development. Ann. Bot. 2011, 107, 1159–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puchooa, D. A simple, rapid and efficient method for the extraction of genomic DNA from lychee (Litchi chinensis Sonn.). Afr. J. Biotechnol. 2004, 3, 253–255. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0. molecular biology and evolution. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [Green Version]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar]
- Choi, Y.; Sims, G.E.; Murphy, S.; Miller, J.R.; Chan, A.P. Predicting the functional effect of amino acid substitutions and indels. PLoS ONE 2012, 7, e46688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Expósito-Rodríguez, M.; Borges, A.A.; Borges-Pérez, A.; Pérez, J.A. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol. 2008, 8, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bemer, M.; Karlova, R.; Ballester, A.R.; Tikunov, Y.M.; Bovy, A.G.; Wolters-Arts, M.; de Barros Rossetto, P.; Angenent, G.C.; de Maagd, R.A. The tomato fruitfull homologs tdr4/ful1 and mbp7/ful2 regulate ethylene-independent aspects of fruit ripening. Plant Cell 2012, 24, 4437–4451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lescot, M. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1953, 226, 497–509. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Chivkunova, O.B.; Merzlyak, M.N.; Reshetnikova, I.V. A spectrophotometric analysis of pigments in apples. Russ. J. Plant Physiol. 2001, 48, 693–700. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. A simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J. Jpn. Soc. Food Sci. Technol. 1992, 39, 925–928. [Google Scholar] [CrossRef] [Green Version]








| Species | Cultivar/Accession | Ripe Fruit Color | NCBI Accession Number | Gene/CDS (bp) | 5′-UTR/Promoter (bp) | Protein (aa) |
|---|---|---|---|---|---|---|
| Extracted from the NCBI database | ||||||
| Solanum lycopersicum L. | cv. Heinz 1706 | Red | NC 015449.3: 67104216–67112934 chromosome 12 Solyc12g098710 | 4008/1110 | 438/1564 | 369 |
| Solanum pimpinellifolium L. | LA0480 | NRDK01042673.1: 47213–54353 Scaffold3068453 | 4009/1110 | 438/1459 | 369 | |
| Solanum pennellii Correll, 1958 | LA0716 | Green | Gene ID: CCXL01009615.1 (3669..9559) | 4006/1110 | 468/1714 | 369 |
| Solanum habrochaites S. Knapp & D. M. Spooner, 1999 | var. LYC4 | Green | Gene ID: CBYS010011028.1 (46212..52055) | 4003/1110 | 473/1307 | 369 |
| Solanum arcanum Peralta | LA2157 | Green | Gene ID: CBYQ010012533.1 (26020..31886) | 4006/1110 | 473/1648 | 369 |
| Identified in this study | ||||||
| S. lycopersicum L. | cv. Heinz (FSCV) | Red | OK318866 | 4007/1110 | 438/1549 | 369 |
| cv. Korneevskii | Red | OK318867 | 4006/1110 | 438/1551 | 369 | |
| cv. Garmoshka | Red | OK318869 | 4009/1110 | 438/1562 | 369 | |
| cv. Malinovyi silach | Red | OK318868 | 4004/1110 | 438/1561 | 369 | |
| cv. Zemba | Red-Violet | OK318871 | 4010/1110 | 438/1560 | 369 | |
| cv. Paul Robeson | Red-Violet | OK318870 | 4009/1110 | 436/1559 | 369 | |
| cv. Black Jack | Red-Violet | OK318872 | 4008/1110 | 436/1560 | 369 | |
| cv. Christmas blueberry | Red-Violet | OK318873 | 4009/1110 | 436/1567 | 369 | |
| cv. Black cherry | Red-Violet | OK318874 | 4008/1110 | 438/1552 | 369 | |
| cv. Cherry zhelto-oranzhevye | Yellow/Orange | OK318875 | 4008/1110 | 438/1548 | 369 | |
| cv. Kopilka zheltaya | Yellow | OK318876 | 4008/1110 | 438/1548 | 369 | |
| cv. Nesozrevayuschii Natasha (NN-25) | Pale Yellow | OK318877 | 4007/1110 | 437/1525 | 369 | |
| S. pimpinellifolium L. | VIR1018 | Red | OK318858 | 4008/1110 | 438/1459 | 369 |
| Solanum cheesmaniae (L. Riley) Fosberg, 1987 | LA0421 | Yellow/Orange | OK318859 | 4010/1110 | 363/1394 | 369 |
| Solanum chilense (Dunal) Reiche | LA2884 | Green | OK318865 | 3999/1110 | 413/1313 | 369 |
| LA1963 | Green | OK318864 | 3994/1110 | 413/1303 | 369 | |
| S. habrochaites S. Knapp & D. M. Spooner, 1999 | LA1777 | Green | OK318860 | 3972/1110 | 474/1398 | 369 |
| LA2144 | Green | OK318861 | 4003/1110 | 473/1293 | 369 | |
| Solanum pennellii Correll, 1958 | LA0716 | Green | OK318863 | 4006/1110 | 468/1714 | 369 |
| LA1926 | Green | OK318862 | 4008/1110 | 468/1714 | 369 | |
| Gene Region/Translated Product | Variability (%) | ||||
|---|---|---|---|---|---|
| Cultivars * | Cultivars and Wild Species * | Wild RF/YF Species * | Wild GF Species * | Wild GF Species ** | |
| Promoter | 6.81 | 17.14 | 3.30 | 9.38 | 7.71 |
| 5′-UTR | 6.47 | 21.50 | 7.31 | 10.23 | 10.86 |
| Gene | 5.92 | 14.89 | 3.85 | 6.22 | 6.67 |
| cDNA | 3.96 | 10.36 | 1.44 | 5.14 | 5.50 |
| Protein | 7.03 | 16.80 | 2.70 | 9.19 | 8.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efremov, G.I.; Shchennikova, A.V.; Kochieva, E.Z. Characterization of 15-cis-ζ-Carotene Isomerase Z-ISO in Cultivated and Wild Tomato Species Differing in Ripe Fruit Pigmentation. Plants 2021, 10, 2365. https://doi.org/10.3390/plants10112365
Efremov GI, Shchennikova AV, Kochieva EZ. Characterization of 15-cis-ζ-Carotene Isomerase Z-ISO in Cultivated and Wild Tomato Species Differing in Ripe Fruit Pigmentation. Plants. 2021; 10(11):2365. https://doi.org/10.3390/plants10112365
Chicago/Turabian StyleEfremov, Gleb I., Anna V. Shchennikova, and Elena Z. Kochieva. 2021. "Characterization of 15-cis-ζ-Carotene Isomerase Z-ISO in Cultivated and Wild Tomato Species Differing in Ripe Fruit Pigmentation" Plants 10, no. 11: 2365. https://doi.org/10.3390/plants10112365