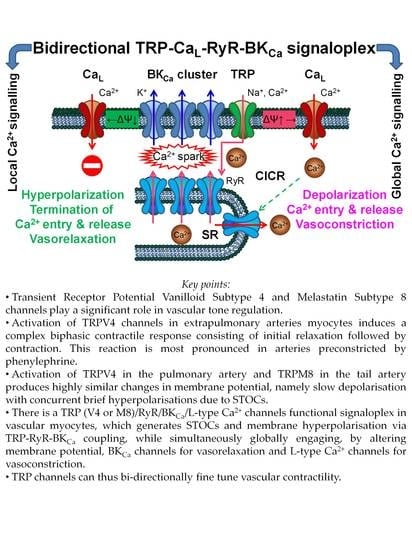
Bidirectional TRP/L Type Ca2+ Channel/RyR/BKCa Molecular and Functional Signaloplex in Vascular Smooth Muscles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Tissue Preparation
2.2. Tensiometry
2.3. Patch-Clamp Recordings
2.4. Intracellular Calcium Recordings
2.5. Statistical Analysis
3. Results
3.1. Contractile Responses to TRPV4 and TRPM8 Activation
3.1.1. The Biphasic Reaction of Pulmonary Artery via TRPV4-Channels after α-Adrenoceptors Activation
3.1.2. Responses of Tail Artery to TRPM8 Agonist Menthol
3.1.3. The Involvement of Other Channels in the Biphasic Action of GSK1016790A
3.2. Membrane Potential Recordings
3.2.1. The Effect of TRPV4 Activation by GSK1016790A on Membrane Potential in Pulmonary Artery Vascular Smooth Muscles Cells (VSMCs)
3.2.2. Membrane Potential Responses to TRPM8 Agonist Menthol in Tail Artery Myocytes
3.3. Effects of TRPM8 and TRPV4 Activation on Ca2+-Activated Membrane Currents in Vascular SMCs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, A.D. Calcium channels in vascular smooth muscle cells. J. Vasc. Res. 1995, 32, 353–370. [Google Scholar] [CrossRef]
- Bolton, T.B.; Prestwich, S.A.; Zholos, A.V.; Gordienko, D.V. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu. Rev. Physiol. 1999, 61, 85–115. [Google Scholar] [CrossRef] [PubMed]
- Zholos, A.V.; Curtis, T.M. TRP Channels in vascular disorders. Curr. Top. Med. Chem. 2013, 13, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhu, Z.; Tepel, M. The role of transient receptor potential channels in metabolic syndrome. Hypertens. Res. 2008, 31, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Heppner, T.J.; Nelson, M.T.; Brayden, J.E. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ. Res. 2005, 97, 1270–1279. [Google Scholar] [CrossRef]
- Dahan, D.; Ducret, T.; Quignard, J.F.; Marthan, R.; Savineau, J.P.; Estève, E. Implication of the ryanodine receptor in TRPV4-induced calcium response in pulmonary arterial smooth muscle cells from normoxic and chronically hypoxic rats. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 303, L824–L833. [Google Scholar] [CrossRef]
- Montell, C. The TRP superfamily of cation channels. Sci. Signal. 2005, 272, re3. [Google Scholar] [CrossRef]
- Wu, L.J.; Sweet, T.B.; Clapham, D.E. International union of basic and clinical pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 2010, 62, 381–404. [Google Scholar] [CrossRef]
- Gees, M.; Owsianik, G.; Nilius, B.; Voets, T. TRP channels. Compr. Physiol. 2012, 2, 563–608. [Google Scholar]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 212–218. [Google Scholar] [CrossRef]
- Gees, M.; Colsoul, B.; Nilius, B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, 1–31. [Google Scholar] [CrossRef]
- Dryn, D.; Melnyk, M.; Kizub, I.; Hu, H.; Soloviev, A.A.I.A.; Zholos, A. The role of Trpv4 cation channels in the regulation of phenylephrine-induced contraction of rat pulmonary arter. Fiziol. Zh. 2016, 62, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Melanaphy, D.; Johnson, C.D.; Kustov, M.V.; Watson, C.A.; Borysova, L.; Burdyga, T.V.; Zholos, A.V. Ion channel mechanisms of rat tail artery contraction-relaxation by menthol involving, respectively, TRPM8 activation and L-type Ca2+ channel inhibition. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1416–H1430. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.; Melanaphy, D.; Purse, A.; Stokesberry, S.A.; Dickson, P.; Zholos, A.V. Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. AJP-Heart Circ. Physiol. 2009, 296, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.R.; Lin, M.J.; McIntosh, L.S.; Sham, J.S.K. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006, 290, L1267–L1276. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.A.; Bell, A.M.; Denis, C.S.; Sali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a andidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Nilius, B.; Vriens, J.; Prenen, J.; Droogmans, G.; Voets, T. TRPV4 Calcium entry channel: A paradigm for gating diversity. AJP-Cell Physiol. 2004, 286, C195–C205. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A Capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef]
- Hemisphere, N.; Recal, C.; The, C.; Clapham, D.E. Hot and cold TRP ion channels. Science 2002, 295, 2228–2229. [Google Scholar]
- Brayden, J.E.; Earley, S.; Nelson, M.T.; Reading, S. Transient receptor potentila (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1116–1120. [Google Scholar] [CrossRef]
- Earley, S.; Brayden, J.E. Transient receptor potential channels and vascular function. Clin. Sci. 2010, 119, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, H.; Tian, W.; Yoshida, K.; Roullet, J.B.; Cohen, D.M. Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation: Src family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. J. Biol. Chem. 2003, 278, 11520–11527. [Google Scholar] [CrossRef]
- Watanabe, H.; Vriens, J.; Suh, S.H.; Benham, C.D.; Droogmans, G.; Nilius, B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J. Biol. Chem. 2002, 277, 47044–47051. [Google Scholar] [CrossRef] [PubMed]
- Vriens, J.; Appendino, G.; Nilius, B. Pharmacology of vanilloid transient receptor potential cation channels. Mol. Pharmacol. 2009, 75, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- White, J.P.M.; Cibelli, M.; Urban, L.; Nilius, B.; McGeown, J.G.; Nagy, I. TRPV4: Molecular conductor of a diverse orchestra. Physiol. Rev. 2016, 96, 911–973. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Varty, L.A.; Rizzo, C.A.; Yang, R.; Correll, C.C.; Phelps, P.T.; Egan, R.W.; Hey, J.A. Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 287, 272–278. [Google Scholar] [CrossRef]
- Yang, H.; Davidson, W.R.; Chambers, C.E.; Pae, W.E.; Sun, B.; Campbell, D.B.; Pu, M. Preoperative pulmonary hypertension is associated with postoperative left ventricular dysfunction in chronic organic mitral regurgitation: An echocardiographic and hemodynamic study. J. Am. Soc. Echocardiogr. 2006, 19, 1051–1055. [Google Scholar] [CrossRef]
- Alessandri-Haber, N.; Dina, O.A.; Yeh, J.J.; Parada, C.A.; Reichling, D.B.; Levine, J.D. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J. Neurosci. 2004, 24, 4444–4452. [Google Scholar] [CrossRef]
- Krüger, J.; Kunert-Keil, C.; Bisping, F.; Brinkmeier, H. Transient receptor potential cation channels in normal and dystrophic mdx muscle. Neuromuscul. Disord. 2008, 18, 501–513. [Google Scholar] [CrossRef]
- Ferna, M.; Nobles, M.; Va, E.; Maxi, M.A.V.; Fernandez-Fernandez, J.M.; Nobles, M.; Currid, A.; Vazquez, E.; Valverde, M.A.; Fernández-Fernández, J.M.; et al. Maxi K+ channel mediates regulatory volume decrease response in a human bronchial epithelial cell line. Am. J. Physiol.-Cell Physiol. 2002, 283, C1705–C1714. [Google Scholar]
- Li, J.; Kanju, P.; Patterson, M.; Chew, W.L.; Cho, S.H.; Gilmour, I.; Oliver, T.; Yasuda, R.; Ghio, A.; Simon, S.A.; et al. TRPV4-mediated calcium influx into human bronchial epithelia upon exposure to diesel exhaust particles. Environ. Health Perspect. 2011, 119, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Casas, S.; Novials, A.; Reimann, F.; Gomis, R.; Gribble, F.M. Calcium elevation in mouse pancreatic beta cells evoked by extracellular human islet amyloid polypeptide involves activation of the mechanosensitive ion channel TRPV4. Diabetologia 2008, 51, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.A.; Skryma, R.; Bidaux, G.; Magleby, K.L.; Scholfeld, C.N.; McGeown, J.G.G.; Prevarskaya, N.; Zholos, A.V.; Fernandez, J.A.; Skryma, R.; et al. Voltage- and cold-dependent gating of single TRPM8 ion channels. J. Gen. Physiol. 2011, 137, 173–195. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, C.; Martín-Martínez, M.; Gómez-Monterrey, I.; González-Muñiz, R. TRPM8 channels: Advances in structural studies and pharmacological modulation. Int. J. Mol. Sci. 2021, 22, 8502. [Google Scholar] [CrossRef]
- Tsavaler, L.; Shapero, M.H.; Morkowski, S.; Laus, R. Trp-P8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001, 61, 3760–3769. [Google Scholar]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 is required for cold sensation in mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef]
- Sabnis, A.S.; Shadid, M.; Yost, G.S.; Reilly, C.A. Human lung epithelial cells express a functional cold-sensing TRPM8 variant. Am. J. Respir. Cell Mol. Biol. 2008, 39, 466–474. [Google Scholar] [CrossRef]
- Stein, R.J.; Santos, S.; Nagatomi, J.; Hayashi, Y.; Minnery, B.S.; Xavier, M.; Patel, A.S.; Nelson, J.B.; Futrell, W.J.; Yoshimura, N.; et al. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J. Urol. 2004, 172, 1175–1178. [Google Scholar] [CrossRef]
- Liu, S.C.; Lu, H.H.; Cheng, L.H.; Chu, Y.H.; Lee, F.P.; Wu, C.C.; Wang, H.W. Identification of the cold receptor TRPM8 in the nasal mucosa. Am. J. Rhinol. Allergy 2015, 29, e112–e116. [Google Scholar] [CrossRef]
- Zholos, A.; Johnson, C.; Burdyga, T.; Melanaphy, D. TRPM channels in the vasculature. Adv. Exp. Med. Biol. 2011, 704, 707–729. [Google Scholar]
- Simonsen, U.; Wandall-Frostholm, C.; Oliván-Viguera, A.; Köhler, R. Emerging roles of calcium-activated K channels and TRPV4 channels in lung oedema and pulmonary circulatory collapse. Acta Physiol. 2017, 219, 176–187. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Qin, L.; Chen, H. Functional cooperation between KCa3.1 and TRPV4 channels in bronchial smooth muscle cell proliferation associated with chronic asthma. Front. Pharmacol. 2017, 8, 559. [Google Scholar] [CrossRef]
- Du, J.; Ma, X.; Shen, B.; Huang, Y.; Birnbaumer, L.; Yao, X. TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. FASEB J. 2014, 28, 4677–4685. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, P.; Li, J.; Lu, J.; Ge, J.; Zhao, Z.; Ma, X.; Wan, S.; Yao, X.; Shen, B. Epoxyeicosatrienoic acids act through TRPV4-TRPC1-KCa1.1 complex to induce smooth muscle membrane hyperpolarization and relaxation in human internal mammary arteries. Biochim. Biophys. Acta 2015, 1852, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qin, F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 2005, 25, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Vanden Abeele, F.; Zholos, A.; Bidaux, G.; Shuba, Y.; Thebault, S.; Beck, B.; Flourakis, M.; Panchin, Y.; Skryma, R.; Prevarskaya, N. Ca2+-independent phospholipase A2-dependent gating of TRPM8 by lysophospholipids. J. Biol. Chem. 2006, 281, 40174–40182. [Google Scholar] [CrossRef]
- Rohács, T.; Lopes, C.M.B.; Michailidis, I.; Logothetis, D.E. PI(4,5)P2 Regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 2005, 8, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.A.; Nash, M.; Bevan, S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J. Neurosci. 2007, 27, 3347–3355. [Google Scholar] [CrossRef]
- Bavencoffe, A.; Kondratskyi, A.; Gkika, D.; Mauroy, B.; Shuba, Y.; Prevarskaya, N.; Skryma, R. Complex regulation of the TRPM8 cold receptor channel: Role of arachidonic acid release following M3 muscarinic receptor stimulation. J. Biol. Chem. 2011, 286, 9849–9855. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Fu, Z.; Hu, J.; Huang, C.; Paudel, O.; Cai, S.; Liedtke, W.; Sham, J.S.K. TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am. J. Physiol.-Cell Physiol. 2013, 305, C704–C715. [Google Scholar] [CrossRef] [PubMed]
- Köhler, R.; Heyken, W.T.; Heinau, P.; Schubert, R.; Si, H.; Kacik, M.; Busch, C.; Grgic, I.; Maier, T.; Hoyer, J.; et al. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Benham, C.D.; Bolton, T.B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J. Physiol. 1986, 381, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Zholos, A.V.; Komori, S.; Ohashi, H.; Bolton, T.B. Ca2+ inhibition of inositol trisphosphate-induced Ca2+ release in single smooth muscle cells of guinea-pig small intestine. J. Physiol. 1994, 481, 97–109. [Google Scholar] [CrossRef]
- Nelson, M.T.; Cheng, H.; Rubart, M.; Santana, L.F.; Bonev, A.D.; Knot, H.J.; Lederer, W.J. Relaxation of arterial smooth muscle by calcium sparks. Science 1995, 270, 633–637. [Google Scholar] [CrossRef]
- Kotecha, N.; Neild, T.O. Effects of denervation on the responses of the rat tail artery to alpha beta-methylene ATP. Gen. Pharmacol. 1987, 18, 535–537. [Google Scholar] [CrossRef]
- Gordienko, D.V.; Zholos, A.V.; Bolton, T.B. Membrane ion channels as physiological targets for local Ca2+ signalling. J. Microsc. 1999, 196, 305–316. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Y.; Bai, S.; Ding, S.; Gao, H.; Yin, S.; Chen, S.; Lu, J.; Wang, H.; Shen, Y.; et al. TRPV4 complexes with the Na+/Ca2+ exchanger and IP3 receptor 1 to regulate local intracellular calcium and tracheal tension in mice. Front. Physiol. 2019, 10, 1471. [Google Scholar] [CrossRef]
- Harper, A.G.S.; Sage, S.O. TRP-Na+/Ca2+ exchanger coupling. In Advances in Experimental Medicine and Biology; Springer New York LLC: New York, NY, USA, 2016; Volume 898, pp. 67–85. [Google Scholar]
- Haustrate, A.; Prevarskaya, N.; Lehen’kyi, V. Role of the TRPV channels in the endoplasmic reticulum calcium homeostasis. Cells 2020, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, M.; Lv, X.; Wang, Z.; Yang, J.; Li, Y.; Yu, F.; Wen, X.; Feng, L.; Zhou, T. Role of transient receptor potential vanilloid 4 in vascular function. Front. Mol. Biosci. 2021, 8, 285. [Google Scholar] [CrossRef] [PubMed]
- Borysova, L.; Dora, K.A.; Garland, C.J.; Burdyga, T. Smooth muscle gap-junctions allow propagation of intercellular Ca2+ waves and vasoconstriction due to Ca2+ based action potentials in rat mesenteric resistance arteries. Cell Calcium 2018, 75, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Filosa, J.A.; Yao, X.; Rath, G. TRPV4 and the regulation of vascular tone. J. Cardiovasc. Pharmacol. 2013, 61, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, N.M.; Wang, L.; Ranke, H.; Liedtke, W.; Tabuchi, A.; Kuebler, W.M. TRPV4 is required for hypoxic pulmonary vasoconstriction. Anesthesiology 2015, 122, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Marziano, C.; Hong, K.; Cope, E.L.; Kotlikoff, M.I.; Isakson, B.E.; Sonkusare, S.K. Nitric oxide-dependent feedback loop regulates transient receptor potential vanilloid 4 (TRPV4) channel cooperativity and endothelial function in small pulmonary arteries. J. Am. Heart Assoc. 2017, 6, e007157. [Google Scholar] [CrossRef]
- Pankey, E.A.; Zsombok, A.; Lasker, G.F.; Kadowitz, P.J. Analysis of responses to the TRPV4 agonist GSK1016790A in the pulmonary vascular bed of the intact-chest rat. Am. J. Physiol.-Heart Circ. Physiol. 2014, 306, 33–40. [Google Scholar] [CrossRef]
- Parpaite, T.; Cardouat, G.; Mauroux, M.; Gillibert-Duplantier, J.; Robillard, P.; Quignard, J.F.; Marthan, R.; Savineau, J.P.; Ducret, T. Effect of hypoxia on TRPV1 and TRPV4 channels in rat pulmonary arterial smooth muscle cells. Pflug. Arch. Eur. J. Physiol. 2016, 468, 111–130. [Google Scholar] [CrossRef]
- Scheraga, R.G.; Southern, B.D.; Grove, L.M.; Olman, M.A. The role of transient receptor potential vanilloid 4 in pulmonary inflammatory diseases. Front. Immunol. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef]
- Zhu, X.; Yao, Y.; Guo, M.; Li, J.; Yang, P.; Xu, H.; Lin, D. Sevoflurane increases intracellular calcium to induce mitochondrial injury and neuroapoptosis. Toxicol. Lett. 2021, 336, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sonkusare, S.K.; Bonev, A.D.; Ledoux, J.; Liedtke, W.; Kotlikoff, M.I.; Heppner, T.J.; Hill-Eubanks, D.C.; Nelson, M.T. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 2012, 336, 597–601. [Google Scholar] [CrossRef]
- Sukumaran, S.V.; Singh, T.U.; Parida, S.; Narasimha, R.C.; Thangamalai, R.; Kandasamy, K.; Singh, V.; Mishra, S.K. TRPV4 channel activation leads to endothelium-dependent relaxation mediated by nitric oxide and endothelium-derived hyperpolarizing factor in rat pulmonary artery. Pharm. Res. 2013, 78, 18–27. [Google Scholar] [CrossRef]
- Thorneloe, K.S.; Sulpizio, A.C.; Lin, Z.; Figueroa, D.J.; Clouse, A.K.; McCafferty, G.P.; Chendrimada, T.P.; Lashinger, E.S.R.; Gordon, E.; Evans, L.; et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-Dichlorophenyl)Sulfonyl]Amino}-3-Hydroxypropanoyl)-1-Piperazinyl]Carbonyl}-3-Methylbutyl)-1-Benzothiophene-2-Carboxamide (GSK1016790A), a novel and potent Transient Receptor Potential Vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J. Pharmacol. Exp. Ther. 2008, 326, 432–442. [Google Scholar] [PubMed]
- Willette, R.N.; Bao, W.; Nerurkar, S.; Yue, T.I.; Doe, C.P.; Stankus, G.; Turner, G.H.; Ju, H.; Thomas, H.; Fishman, C.E.; et al. Systemic activation of the Transient Receptor Potential Vanilloid Subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J. Pharmacol. Exp. Ther. 2008, 326, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Qian, A.; Oetjen, L.K.; Yu, W.; Yang, P.; Feng, J.; Xie, Z.; Liu, S.; Yin, S.; Dryn, D.; et al. TRPV4 channel signaling in macrophages promotes gastrointestinal motility via direct effects on smooth muscle cells. Immunity 2018, 49, 107–119.e4. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Du, L.; Liu, S.; Lan, Z.; Zang, K.; Feng, J.; Zhao, Y.; Yang, X.; Xie, Z.; Wang, P.L.; et al. A TRPV4-dependent neuroimmune axis in the spinal cord promotes neuropathic pain. J. Clin. Investig. 2023, 133, e161507. [Google Scholar] [CrossRef]
- Wu, Q.F.; Qian, C.; Zhao, N.; Dong, Q.; Li, J.; Wang, B.; Chen, L.; Yu, L.; Han, B.; Du, Y.M.; et al. Activation of Transient Receptor Potential Vanilloid 4 involves in hypoxia/reoxygenation injury in cardiomyocytes. Cell Death Dis. 2017, 8, e2828. [Google Scholar] [CrossRef]
- Lawhorn, B.G.; Brnardic, E.J.; Behm, D.J. Recent advances in TRPV4 agonists and antagonists. Bioorg. Med. Chem. Lett. 2020, 30, 127022. [Google Scholar] [CrossRef]
- Ai, C.; Wang, Z.; Li, P.; Wang, M.; Zhang, W.; Song, H.; Cai, X.; Lv, K.; Chen, X.; Zheng, Z. Discovery and pharmacological characterization of a novel benzimidazole TRPV4 antagonist with cyanocyclobutyl moiety. Eur. J. Med. Chem. 2023, 249, 115137. [Google Scholar] [CrossRef]
- Everaerts, W.; Zhen, X.; Ghosh, D.; Vriens, J.; Gevaert, T.; Gilbert, J.P.; Hayward, N.J.; McNamara, C.R.; Xue, F.; Moran, M.M.; et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc. Natl. Acad. Sci. USA 2010, 107, 19084–19089. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Cope, E.L.; DeLalio, L.J.; Marziano, C.; Isakson, B.E.; Sonkusare, S.K. TRPV4 (transient receptor potential vanilloid 4) channel-dependent negative feedback mechanism regulates Gq protein-coupled receptor-induced vasoconstriction. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 542–554. [Google Scholar] [CrossRef]
- Inoue, R.; Okada, T.; Onoue, H.; Hara, Y.; Shimizu, S.; Naitoh, S.; Ito, Y.; Mori, Y. The Transient Receptor Potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ. Res. 2001, 88, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Daneva, Z.; Kuppusamy, M.; Ottolini, M.; Baker, T.M.; Klimentova, E.; Shah, S.A.; Sokolowski, J.D.; Park, M.S.; Sonkusare, S.K. Novel smooth muscle Ca2+-signaling nanodomains in blood pressure regulation. Circulation 2022, 146, 548–564. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dryn, D.O.; Melnyk, M.I.; Melanaphy, D.; Kizub, I.V.; Johnson, C.D.; Zholos, A.V. Bidirectional TRP/L Type Ca2+ Channel/RyR/BKCa Molecular and Functional Signaloplex in Vascular Smooth Muscles. Biomolecules 2023, 13, 759. https://doi.org/10.3390/biom13050759
Dryn DO, Melnyk MI, Melanaphy D, Kizub IV, Johnson CD, Zholos AV. Bidirectional TRP/L Type Ca2+ Channel/RyR/BKCa Molecular and Functional Signaloplex in Vascular Smooth Muscles. Biomolecules. 2023; 13(5):759. https://doi.org/10.3390/biom13050759
Chicago/Turabian StyleDryn, Dariia O., Mariia I. Melnyk, Donal Melanaphy, Igor V. Kizub, Christopher D. Johnson, and Alexander V. Zholos. 2023. "Bidirectional TRP/L Type Ca2+ Channel/RyR/BKCa Molecular and Functional Signaloplex in Vascular Smooth Muscles" Biomolecules 13, no. 5: 759. https://doi.org/10.3390/biom13050759