Infection Rate of Respiratory Viruses in the Pandemic SARS-CoV-2 Period Considering Symptomatic Patients: Two Years of Ongoing Observations
Abstract
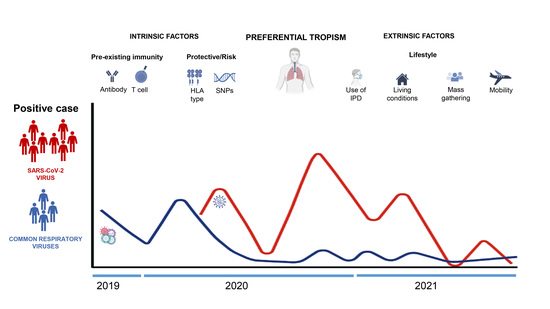
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. FilmArray Respiratory Panel 2.1plus
2.3. SARS-CoV-2 Real-Time Qualitative PCR
Government Measures against SARS-CoV-2 Spread
2.4. Statistical Analyses
3. Results
3.1. Clinical and Epidemiological Features of Enrolled Patients after FilmArray Respiratory Panel 2.1plus Assay
3.2. Profiles of Respiratory Viruses and SARS-CoV-2 Detected in the Enrolled Patients
3.3. The Comparison between Percentage of Positive Cases in the Last 4 Years (2018–2021) Confirms the Influence of SARS-CoV-2
3.4. SARS-CoV-2 Positivity before and after Vaccination Campaigns
3.5. Laboratory Features of COVID-19 Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Si, Y.; Zhao, Z.; Chen, R.; Zhong, H.; Liu, T.; Wang, M.; Song, X.; Li, W.; Ying, B. Epidemiological surveillance of common respiratory viruses in patients with suspected COVID-19 in Southwest China. BMC Infect Dis. 2020, 20, 688. [Google Scholar] [CrossRef] [PubMed]
- Mattana, G.; Albitar-Nehme, S.; Cento, V.; Colagrossi, L.; Piccioni, L.; Raponi, M.; Raucci, U.; Vittucci, A.C.; Reale, A.; Villani, A.; et al. Back to the future (of common respiratory viruses). J. Glob. Antimicr. Resist. 2022, 28, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Gatineau, A.; Kaba, L.; Boschi, C.; Devaux, C.; Casalta, J.P.; Gautret, P.; Chaudet, H.; Colson, P.; Raoult, D. Control of common viral epidemics but not of SARS-CoV-2 through the application of hygiene and distancing measures. J. Clin. Virol. 2022, 150–151, 105163. [Google Scholar] [CrossRef] [PubMed]
- Asner, S.A.; Science, M.E.; Tran, D.; Smieja, M.; Merglen, A.; Mertz, D. Clinical disease severity of respiratory viral co-infection versus single viral infection: A systematic review and meta-analysis. PLoS ONE 2014, 9, e99392. [Google Scholar] [CrossRef] [Green Version]
- Glezen, W.P. Emerging infections: Pandemic influenza. Epidemiol. Rev. 1996, 18, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Finianos, M.; Issa, R.; Curran, M.D.; Afif, C.; Rajab, M.; Irani, J.; Hakimeh, N.; Naous, A.; Hajj, M.J.; Hajj, P.; et al. Etiology, seasonality, and clinical characterization of viral respiratory infections among hospitalized children in Beirut, Lebanon. J. Med. Virol. 2016, 88, 1874–1881. [Google Scholar] [CrossRef]
- Itaya, T.; Furuse, Y.; Jindai, K. Does COVID-19 infection impact on the trend of seasonal influenza infection? 11 countries and regions, from 2014 to 2020. Int. J. Infect. Dis. 2020, 97, 78–80. [Google Scholar] [CrossRef]
- Reperant, L.A.; Kuiken, T.; Osterhaus, A.D. Influenza viruses: From birds to humans. Hum. Vaccin. Immunother. 2012, 8, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.; Paulson, J.C. Adaptation of influenza viruses to human airway receptors. J. Biol. Chem. 2021, 296, 100017. [Google Scholar] [CrossRef]
- Branche, A.R.; Falsey, A.R. Parainfluenza Virus Infection. Semin. Resp. Crit. Care Med. 2016, 37, 538–554. [Google Scholar] [CrossRef]
- Anderson, T.K.; Macken, C.A.; Lewis, N.S.; Scheuermann, R.H.; Van Reeth, K.; Brown, I.H.; Swenson, S.L.; Simon, G.; Saito, T.; Berhane, Y.; et al. A Phylogeny-Based Global Nomenclature System and Automated Annotation Tool for H1 Hemagglutinin Genes from Swine Influenza A Viruses. mSphere 2016, 1, e00275-16. [Google Scholar] [CrossRef] [Green Version]
- Henrickson, K.J.; Kuhn, S.M.; Savatski, L.L. Epidemiology and cost of infection with human parainfluenza virus types 1 and 2 in young children. Clin. Inf. Dis. 1994, 18, 770–779. [Google Scholar] [CrossRef]
- Eveno, T.; Dirr, L.; El-Deeb, I.M.; Guillon, P.; von Itzstein, M. Targeting Human Parainfluenza Virus Type-1 Haemagglutinin-Neuraminidase with Mechanism-Based Inhibitors. Viruses 2019, 11, 417. [Google Scholar] [CrossRef] [Green Version]
- Wendt, C.H.; Weisdorf, D.J.; Jordan, M.C.; Balfour, H.H., Jr.; Hertz, M.I. Parainfluenza virus respiratory infection after bone marrow transplantation. N. Engl. J. Med. 1992, 326, 921–926. [Google Scholar] [CrossRef]
- Chemaly, R.F.; Ghosh, S.; Bodey, G.P.; Rohatgi, N.; Safdar, A.; Keating, M.J.; Champlin, R.E.; Aguilera, E.A.; Tarrand, J.J.; Raad, I.I. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: A retrospective study at a major cancer center. Medicine 2006, 85, 278–287. [Google Scholar] [CrossRef]
- Takeda, M.; Shirogane, Y. Human Metapneumovirus Infection. Rinsho Byori 2016, 64, 1057–1064. [Google Scholar]
- Lê, V.B.; Dubois, J.; Couture, C.; Cavanagh, M.H.; Uyar, O.; Pizzorno, A.; Rosa-Calatrava, M.; Hamelin, M.; Boivin, G. Human metapneumovirus activates NOD-like receptor protein 3 inflammasome via its small hydrophobic protein which plays a detrimental role during infection in mice. PLoS Path 2019, 15, e1007689. [Google Scholar] [CrossRef]
- Ballegeer, M.; Saelens, X. Cell-Mediated Responses to Human Metapneumovirus Infection. Viruses 2020, 12, 542. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Boyapalle, S. Epidemiologic, experimental, and clinical links between respiratory syncytial virus infection and asthma. Clin. Microbiol. Rev. 2008, 21, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Shang, Z.; Tan, S.; Ma, D. Respiratory syncytial virus: From pathogenesis to potential therapeutic strategies. Int. J. Biol. Sci. 2021, 17, 4073–4091. [Google Scholar] [CrossRef]
- Hamre, D.; Procknow, J.J. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 1966, 121, 190–193. [Google Scholar] [CrossRef]
- van der Hoek, L.; Pyrc, K.; Jebbink, M.F.; Vermeulen-Oost, W.; Berkhout, R.J.; Wolthers, K.C.; Wertheim-van Dillen, P.M.; Kaandorp, J.; Spaargaren, J.; Berkhout, B. Identification of a new human coronavirus. Nat. Med. 2004, 10, 368–373. [Google Scholar] [CrossRef]
- Drosten, C.; Günther, S.; Preiser, W.; van der Werf, S.; Brodt, H.R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.; et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef]
- Kumar, V. Emerging Human Coronavirus Infections (SARS, MERS, and COVID-19): Where They Are Leading Us. Rev. Immunol. 2021, 40, 5–53. [Google Scholar] [CrossRef]
- Mohamadian, M.; Chiti, H.; Shoghli, A.; Biglari, S.; Parsamanesh, N.; Esmaeilzadeh, A. COVID-19: Virology, biology and novel laboratory diagnosis. J. Gen. Med. 2021, 23, e3303. [Google Scholar] [CrossRef]
- Ciotti, M.; Ciccozzi, M.; Pieri, M.; Bernardini, S. The COVID-19 pandemic: Viral variants and vaccine efficacy. Crit. Rev. Clin. Lab. Sci. 2022, 59, 66–75. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar]
- Yu, J.; Xie, Z.; Zhang, T.; Lu, Y.; Fan, H.; Yang, D.; Bénet, T.; Vanhems, P.; Shen, K.; Huang, F.; et al. Comparison of the prevalence of respiratory viruses in patients with acute respiratory infections at different hospital settings in North China, 2012–2015. BMC Infect. Dis. 2018, 18, 72. [Google Scholar] [CrossRef] [Green Version]
- EckoE, J.; Locher, K.; Caza, M.; Li, L.; Lavergne, V.; Charles, M. Evaluation of the BioFire® COVID-19 test and Respiratory Panel 2.1 for rapid identification of SARS-CoV-2 in nasopharyngeal swab samples. Diagn. Microbiol. Infect. Dis. 2021, 99, 115260. [Google Scholar]
- Nuccetelli, M.; Pieri, M.; Grelli, S.; Ciotti, M.; Miano, R.; Andreoni, M.; Bernardini, S. SARS-CoV-2 infection serology: A useful tool to overcome lockdown? Cell Death Discov. 2020, 6, 38. [Google Scholar] [CrossRef]
- Costanza, G.; Doldo, E.; Ferlosio, A.; Tarquini, C.; Passeri, D.; Cascella, R.; Bavetta, M.; Di Stefani, A.; Bonifati, C.; Agostinelli, S.; et al. Expression and potential role of cellular retinol binding protein I in psoriasis. Oncotarget 2018, 9, 36736–36749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, K.H.; Lee, S.W.; Kim, T.S.; Huh, H.J.; Lee, J.; Kim, S.Y.; Park, J.-S.; Kim, G.J.; Sung, H.; Roh, K.H.; et al. Guidelines for Laboratory Diagnosis of Coronavirus Disease 2019 (COVID-19) in Korea. Ann. Lab. Med. 2020, 40, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Morales-Morales, C.; González-Urquijo, M.; Barahona-Iglesias, A.J.; Abdo-Francis, J.M.; Sobrino-Cossío, S.R.; Bosques-Padilla, F.J. Recomendaciones para procedimientos quirúrgicos durante la pandemia de COVID-19 (SARS-CoV-2). Liderazgo hacia el retorno a lo electivo. Cir. Cir. 2020, 88, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Margallo, L.N.; Diaz, M.; Lim, P.P. 2019 Novel Coronavirus Pandemic: What Do We Know? Sou. Dak. Med. 2020, 73, 262–264. [Google Scholar]
- Matteucci, C.; Minutolo, A.; Balestrieri, E.; Petrone, V.; Fanelli, M.; Malagnino, V.; Ianetta, M.; Giovinazzo, A.; Barreca, F.; Di Cesare, S.; et al. Thymosin Alpha 1 Mitigates Cytokine Storm in Blood Cells From Coronavirus Disease 2019 Patients. Open Forum Infect Dis. 2021, 8, ofaa588. [Google Scholar] [CrossRef]
- Pieri, M.; Nuccetelli, M.; Nicolai, E.; Sarubbi, S.; Grelli, S.; Bernardini, S. Clinical validation of a second generation anti-SARS-CoV-2 IgG and IgM automated chemiluminescent immunoassay. J. Med. Virol. 2021, 93, 2523–2528. [Google Scholar] [CrossRef]
- Novelli, G.; Liu, J.; Biancolella, M.; Alonzi, T.; Novelli, A.; Patten, J.J.; Cocciadiferro, D.; Agolini, E.; Colona, V.L.; Rizzacasa, B.; et al. Inhibition of HECT E3 ligases as potential therapy for COVID-19. Cell Death Dis. 2021, 12, 310. [Google Scholar] [CrossRef]
- Balestrieri, E.; Minutolo, A.; Petrone, V.; Fanelli, M.; Iannetta, M.; Malagnino, V.; Zordan, M.; Vitale, P.; Charvet, B.; Horvat, B.; et al. Evidence of the pathogenic HERV-W envelope expression in T lymphocytes in association with the respiratory outcome of COVID-19 patients. eBioMedicine 2021, 66, 103341. [Google Scholar] [CrossRef]
- Tu, H.; Tu, S.; Gao, S.; Shao, A.; Sheng, J. Current epidemiological and clinical features of COVID-19; a global perspective from China. In The COVID-19 Reader, 1st ed.; Routledge: London, UK, 2020; pp. 53–72. [Google Scholar]
- Campione, E.; Cosio, T.; Rosa, L.; Lanna, C.; Di Girolamo, S.; Gaziano, R.; Valenti, P.; Bianchi, L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int. J. Mol. Sci. 2020, 21, 4903. [Google Scholar] [CrossRef]
- Amati, F.; Vancheri, C.; Latini, A.; Colona, V.L.; Grelli, S.; D’Apice, M.R.; Balestrieri, E.; Passarelli, C.; Minutolo, A.; Loddo, S.; et al. Expression profiles of the SARS-CoV-2 host invasion genes in nasopharyngeal and oropharyngeal swabs of COVID-19 patients. Heliyon 2020, 6, e05143. [Google Scholar] [CrossRef]
- Novelli, A.; Biancolella, M.; Borgiani, P.; Cocciadiferro, D.; Colona, V.L.; D’Apice, M.R.; Rogliani, P.; Zaffina, S.; Leonardis, F.; Campana, A.; et al. Analysis of ACE2 genetic variants in 131 Italian SARS-CoV-2-positive patients. Hum. Gen. 2020, 14, 29. [Google Scholar] [CrossRef]
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 2020, 11, 55290. [Google Scholar] [CrossRef]
- Bulfone, T.C.; Malekinejad, M.; Rutherford, G.W.; Razani, N. Outdoor Transmission of SARS-CoV-2 and Other Respiratory Viruses: A Systematic Review. J. Infect. Dis. 2021, 223, 550–561. [Google Scholar] [CrossRef]
- Olsen, S.J.; Azziz-Baumgartner, E.; Budd, A.P.; Brammer, L.; Sullivan, M.; Pineda, R.F.; Cohen, C.; Fry, A.M. Decreased Influenza Activity During the COVID-19 Pandemic—United States, Australia, Chile, and South Africa, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1305–1309. [Google Scholar] [CrossRef]
- Mostafa, H.H.; Carroll, K.C.; Hicken, R.; Berry, G.J.; Manji, R.; Smith, E.; Rakeman, J.L.; Fowler, R.C.; Leelawong, M.; Butler-Wu, S.M.; et al. Multicenter Evaluation of the Cepheid Xpert Xpress SARS-CoV-2/Flu/RSV Test. J. Clin. Microbiol. 2021, 59, e02955-20. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Oduyebo, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, J.F., III; Azziz-Baumgartner, E.; Gilboa, S.M.; et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1641–1647. [Google Scholar] [CrossRef]
- Ouyang, S.M.; Zhu, H.Q.; Xie, Y.N.; Zou, Z.S.; Zuo, H.M.; Rao, Y.W.; Liu, X.Y.; Zhong, B.; Chen, X. Temporal changes in laboratory markers of survivors and non-survivors of adult inpatients with COVID-19. BMC Infec. Dis. 2020, 20, 952. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [Green Version]




| Viruses | Bacteria |
|---|---|
| Adenovirus Coronaviruses (229E, HKU1, OC43, NL63) Middle East Respiratory Syndrome Coronavirus (Mers-CoV) Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Human Metapneumovirus (HMPV) Human Rhinovirus/Enterovirus Influenza A (H1, H1 2099, H3) Influenza B Parainfluenzavirus (PIV) 1,2,3,4 Respiratory Syncytial Virus (RSV) | Bordetella pertussis Bordetella parapertussis Chlamydophila pneumoniae Mycoplasma pneumoniae |
| Number of Samples | 2300 | ||
|---|---|---|---|
| Age | 65.4 ± 16.7 | number | % |
| Sex | Male | 1540 | 66.9 |
| Female | 760 | 33.10 | |
| Type of Infection | |||
| SARS-CoV-2 | Positive Cases | 707 | 31.06 |
| Adenovirus | Positive Cases | 1 | 0.043 |
| Influenza viruses | Positive Cases | 43 | 1.85 |
| Syncytial Respiratory Virus | Positive Cases | 28 | 1.20 |
| Metapneumoviruses | Positive Cases | 7 | 0.30 |
| Parainfluenza viruses | Positive Cases | 13 | 0.56 |
| Coronaviruses | Positive Cases | 61 | 2.60 |
| Negative Cases | 1447 | 62.34 |
| Type | Co-Infectious Pathogens | Number of Cases |
|---|---|---|
| InfA + HCoV | 2 | 1 |
| InfA + HPIV | 2 | 1 |
| InfA + SARS-CoV-2 | 2 | 1 |
| HSRV + HCoV | 2 | 2 |
| HSRV + SARS-CoV-2 | 2 | 2 |
| HMPV + SARS-CoV-2 | 1 | 1 |
| InfA + InfB + HCoV | 3 | 1 |
| Age Range | Positive SARS-CoV-2 Detection Until December 2020 | Positive SARS-CoV-2 Detection Until November 2021 | |
|---|---|---|---|
| % (n. pos/n. tot) | % (n. pos/n. tot) | p < Value (Chi2 Test) | |
| 18–20 | 25 (1/4) | 5.5 (1/18) | 0.85 |
| 21–30 | 18.76 (6/32) | 3.9 (2/52) | 0.02 |
| 31–40 | 36.84 (21/57) | 15.6 (10/64) | 0.001 |
| 41–50 | 46.93 (46/98) | 27.17 (25/92) | 0.004 |
| 51–60 | 54–6 (95/174) | 29–93 (44/147) | 0.00007 |
| 61–70 | 50.76 (100/197) | 27.9 (60/215) | 0.00001 |
| 71–80 | 40 (100/240) | 25.79 (57/221) | 0.0003 |
| 81–90 | 44.84 (94/194) | 19.01 (31/163) | 0.00001 |
| 91–98 | 31.03 (12/29) | 11.07 (2/17) | 0.03 |
| Biochemical Data | SARS-CoV-2-Positive Cases (n = 444) | |
|---|---|---|
| Reference Values | (Mean Values ± SE) | |
| Hematocrit | 38–54 | 38.05 ± 0.8 |
| Hemoglobin (g/L) | 12.5–18 | 12.78 ± 1.2 |
| Erythrocyte (×1012/L) | 4–5,700,000 | 4.39 ± 0.27 |
| Platelets (×109/L) | 150–450 | 226.61 ± 21.1 |
| Leucocytes (×109/L) | 4–100,000 | 6.89 ± 2.5 |
| Lymphocytes (%) | 12–50.0 | 17.59 ± 4.67 |
| Basophils (%) | 0–2 | 0.3 ± 0.15 |
| Neutrophils (%) | 37–75 | 75.85 ± 3.6 |
| Eosinophils (%) | 0–7 | 0.62 ± 0.16 |
| Monocytes (%) | 3–12.0 | 6.67 ± 0.78 |
| Azotemia (mg/dL) | 15–50 | 51.2 ± 34.7 |
| HDL-CHOL (mg/dL) | 45–65 | 29.59 ± 5.32 |
| CHOL (mg/dL) | 160–220 | 147.89 ± 17.4 |
| APTT—Seconds | 20–40 | 30.77 ± 5.28 |
| ATIII—Antitrombin (%) | 75–129 | 119.78 ± 20.85 |
| Fibrinogen (mg/dL) | 200–400 | 568.8 ± 105.29 |
| Gamma GT | 8–61.0 | 47.89 ± 18.61 |
| GOT/AST (U/L) | 0–45.0 | 41.44 ± 9.78 |
| GPT/ALT (U/L) | 0–50.0 | 31.82 ± 22.12 |
| LDH (IU/L) | 122–222 | 316.3 ± 75.47 |
| MCH (FL) | 78–98 | 29.1 ± 1.03 |
| MCHC (g/dL) | 30–36 | 33.45 ± 0.87 |
| MCV | 80–96.0 | 85.9 ± 3.81 |
| CRP (mg/L) | 5–6.0; 500–1.000 # | 89.74 ± 29.79 |
| PT (%) | 80–120 | 82.71 ± 12.36 |
| TG (mg/dL) | 50–155 | 119.21 ± 23.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanza, G.; Paba, P.; Ciotti, M.; Ombres, D.; Di Carlo, S.; Marcuccilli, F.; Bertoli, A.; Di Traglia, L.; Mozzani, M.; Piredda, L.; et al. Infection Rate of Respiratory Viruses in the Pandemic SARS-CoV-2 Period Considering Symptomatic Patients: Two Years of Ongoing Observations. Biomolecules 2022, 12, 987. https://doi.org/10.3390/biom12070987
Costanza G, Paba P, Ciotti M, Ombres D, Di Carlo S, Marcuccilli F, Bertoli A, Di Traglia L, Mozzani M, Piredda L, et al. Infection Rate of Respiratory Viruses in the Pandemic SARS-CoV-2 Period Considering Symptomatic Patients: Two Years of Ongoing Observations. Biomolecules. 2022; 12(7):987. https://doi.org/10.3390/biom12070987
Chicago/Turabian StyleCostanza, Gaetana, Pierpaolo Paba, Marco Ciotti, Domenico Ombres, Stefano Di Carlo, Fabbio Marcuccilli, Ada Bertoli, Loide Di Traglia, Marcello Mozzani, Lucia Piredda, and et al. 2022. "Infection Rate of Respiratory Viruses in the Pandemic SARS-CoV-2 Period Considering Symptomatic Patients: Two Years of Ongoing Observations" Biomolecules 12, no. 7: 987. https://doi.org/10.3390/biom12070987