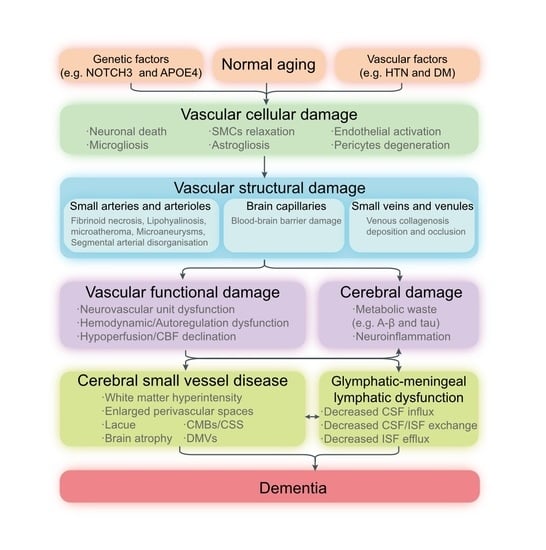
The Underlying Role of the Glymphatic System and Meningeal Lymphatic Vessels in Cerebral Small Vessel Disease
Abstract
:1. Introduction
2. An Overall Perspective of the Glymphatic System and Meningeal Lymphatic Vessels
2.1. The Cerebrospinal Fluid Circulation
2.2. Virchow-Robin Spaces and the Glymphatic System
2.3. Meningeal Lymphatic Vessel
2.4. The Interaction between the Glymphatic System and Meningeal Lymphatic Vessels
2.5. Meningeal and Skull Bone Marrow Immunity
3. The Underlying Role of the Glymphatic and Meningeal Lymphatic System in CSVD
3.1. Common Vascular Risk Factors
3.1.1. Glymphatic-Meningeal Lymphatic Dysfunction in Aging
3.1.2. Glymphatic-Meningeal Lymphatic Dysfunction in Hypertension
3.1.3. Glymphatic-Meningeal Lymphatic Dysfunction in Diabetes
3.1.4. Glymphatic-Meningeal Lymphatic Dysfunction in Lipids Metabolism
3.2. Mechanisms Linking Glymphatic and Meningeal Lymphatic System to CSVD
3.2.1. Neurovascular Unit Dysfunction and Neurovascular Coupling Dysfunction
3.2.2. Blood-Brain Barrier Damage
3.2.3. Neuroinflammation
3.2.4. Proteostasis and Protein Aggregation
3.2.5. Chronic Hypoperfusion and Cerebral Blood Flow Reduction
3.2.6. Hemodynamic Dysfunction
3.2.7. Venous Collagenosis
3.3. Subtype of Cerebral Small Vessel Disease
3.3.1. Amyloid-Related Cerebral Small Vessel Disease
3.3.2. Arteriolosclerosis Cerebral Small Vessel Disease
3.3.3. Microinfarcts
3.4. Cognitive Impairment and Dementia: A Common Final Pathway
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nam, K.; Kwon, H.; Lim, J.; Han, M.; Nam, H.; Lee, Y. The presence and severity of cerebral small vessel disease increases the frequency of stroke in a cohort of patients with large artery occlusive disease. PLoS ONE 2017, 12, e184944. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, F.E.; de Groot, J.C.; Achten, E.; Oudkerk, M.; Ramos, L.M.; Heijboer, R.; Hofman, A.; Jolles, J.; van Gijn, J.; Breteler, M.M. Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The Rotterdam Scan Study. J. Neurol. Neurosurg. Psychiatry 2001, 70, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Shi, Y.; Wardlaw, J.M. Update on cerebral small vessel disease: A dynamic whole-brain disease. BMJ 2016, 1, 83–92. [Google Scholar] [CrossRef]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Plog, B.A.; Nedergaard, M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 379–394. [Google Scholar] [CrossRef]
- Hannawi, Y.; Caceres, E.; Ewees, M.G.; Powell, K.A.; Bratasz, A.; Schwab, J.M.; Rink, C.L.; Zweier, J.L. Characterizing the Neuroimaging and Histopathological Correlates of Cerebral Small Vessel Disease in Spontaneously Hypertensive Stroke-Prone Rats. Front. Neurol. 2021, 12, 740298. [Google Scholar] [CrossRef]
- Koundal, S.; Elkin, R.; Nadeem, S.; Xue, Y.; Constantinou, S.; Sanggaard, S.; Liu, X.; Monte, B.; Xu, F.; Van Nostrand, W.; et al. Optimal Mass Transport with Lagrangian Workflow Reveals Advective and Diffusion Driven Solute Transport in the Glymphatic System. Sci. Rep. 2020, 10, 1990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, Y.; Wang, J.; Gong, X.; Chen, Z.; Zhang, X.; Cai, J.; Chen, S.; Fang, L.; Sun, J.; et al. Glymphatic clearance function in patients with cerebral small vessel disease. NeuroImage 2021, 238, 118257. [Google Scholar] [CrossRef]
- Pollay, M. The function and structure of the cerebrospinal fluid outflow system. Cereb. Fluid Res. 2010, 7, 9. [Google Scholar] [CrossRef]
- Biceroglu, H.; Albayram, S.; Ogullar, S.; Hasiloglu, Z.I.; Selcuk, H.; Yuksel, O.; Karaaslan, B.; Yildiz, C.; Kiris, A. Direct venous spinal reabsorption of cerebrospinal fluid: A new concept with serial magnetic resonance cisternography in rabbits. J. Neurosurg. Spine 2012, 16, 394–401. [Google Scholar] [CrossRef]
- Proulx, S.T. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell. Mol. Life Sci. 2021, 78, 2429–2457. [Google Scholar] [CrossRef]
- Kida, S.; Pantazis, A.; Weller, R.O. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol. 1993, 19, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.G.; Manzo, R.P.; Fenstermacher, J.D.; Potts, D.G. Cerebrospinal fluid absorption in the rabbit. Optic pathways. Graefes Arch. Clin. Exp. Ophthalmol. 1988, 226, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, A.; Papaiconomou, C.; Djenic, J.; Midha, R.; Johnston, M. Lymphatic cerebrospinal fluid absorption pathways in neonatal sheep revealed by subarachnoid injection of Microfil. Neuropathol. Appl. Neurobiol. 2003, 29, 563–573. [Google Scholar] [CrossRef]
- Ma, Q.; Ineichen, B.V.; Detmar, M.; Proulx, S.T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 2017, 8, 1413–1434. [Google Scholar] [CrossRef]
- Brown, R.; Benveniste, H.; Black, S.E.; Charpak, S.; Dichgans, M.; Joutel, A.; Nedergaard, M.; Smith, K.J.; Zlokovic, B.V.; Wardlaw, J.M. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc. Res. 2018, 114, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Woollam, D.H.; Millen, J.W. The perivascular spaces of the mammalian central nervous system and their relation to the perineuronal and subarachnoid spaces. J. Anat. 1955, 89, 193–200. [Google Scholar] [PubMed]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J.; et al. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef]
- Bucchieri, F.; Farina, F.; Zummo, G.; Cappello, F. Lymphatic vessels of the dura mater: A new discovery? J. Anat. 2015, 227, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Lukic, I.K.; Gluncic, V.; Ivkic, G.; Hubenstorf, M.; Marusic, A. Virtual dissection: A lesson from the 18th century. Lancet 2003, 362, 2110–2113. [Google Scholar] [CrossRef]
- Foldi, M.; Gellert, A.; Kozma, M.; Poberai, M.; Zoltan, O.T.; Csanda, E. New contributions to the anatomical connections of the brain and the lymphatic system. Acta Anat. 1966, 64, 498–505. [Google Scholar] [CrossRef]
- Absinta, M.; Ha, S.; Nair, G.; Sati, P.; Luciano, N.J.; Palisoc, M.; Louveau, A.; Zaghloul, K.A.; Pittaluga, S.; Kipnis, J.; et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. eLife 2017, 6, e29738. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Cho, H.; Kim, J.; Kim, S.H.; Ham, J.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.; Hong, Y.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, P.; Kedra, A.; Shotar, E.; Bonnin, S.; Boch, A.L.; Shor, N.; Clarencon, F.; Touitou, V.; Lenck, S. Idiopathic Intracranial Hypertension: Glymphedema of the Brain. J. Neuroophthalmol. 2021, 41, 93–97. [Google Scholar] [CrossRef]
- Raper, D.; Louveau, A.; Kipnis, J. How Do Meningeal Lymphatic Vessels Drain the CNS? Trends Neurosci. 2016, 39, 581–586. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, J.; Zhang, W.; Gong, X.; Yan, S.; Zhang, K.; Luo, Z.; Sun, J.; Jiang, Q.; Lou, M. Impairment of the Glymphatic Pathway and Putative Meningeal Lymphatic Vessels in the Aging Human. Ann. Neurol. 2020, 87, 357–369. [Google Scholar] [CrossRef]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.C.; Kingsmore, K.M.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018, 560, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ries, M.; Decker, Y.; Müller, A.; Riner, C.; Bücker, A.; Fassbender, K.; Detmar, M.; Proulx, S.T. Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol. 2019, 137, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Cugurra, A.; Mamuladze, T.; Rustenhoven, J.; Dykstra, T.; Beroshvili, G.; Greenberg, Z.J.; Baker, W.; Papadopoulos, Z.; Drieu, A.; Blackburn, S.; et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science 2021, 373, eabf7844. [Google Scholar] [CrossRef] [PubMed]
- Brioschi, S.; Wang, W.; Peng, V.; Wang, M.; Shchukina, I.; Greenberg, Z.J.; Bando, J.K.; Jaeger, N.; Czepielewski, R.S.; Swain, A.; et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science 2021, 373, eabf9277. [Google Scholar] [CrossRef] [PubMed]
- Schafflick, D.; Wolbert, J.; Heming, M.; Thomas, C.; Hartlehnert, M.; Börsch, A.; Ricci, A.; Martín-Salamanca, S.; Li, X.; Lu, I.; et al. Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat. Neurosci. 2021, 24, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Price, T.T.; Cantelli, G.; Ngo, B.; Warner, M.J.; Olivere, L.; Ridge, S.M.; Jablonski, E.M.; Therrien, J.; Tannheimer, S.; et al. Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature 2018, 560, 55–60. [Google Scholar] [CrossRef]
- Alves De Lima, K.; Rustenhoven, J.; Da Mesquita, S.; Wall, M.; Salvador, A.F.; Smirnov, I.; Martelossi Cebinelli, G.; Mamuladze, T.; Baker, W.; Papadopoulos, Z.; et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat. Immunol. 2020, 21, 1421–1429. [Google Scholar] [CrossRef]
- Herisson, F.; Frodermann, V.; Courties, G.; Rohde, D.; Sun, Y.; Vandoorne, K.; Wojtkiewicz, G.R.; Masson, G.S.; Vinegoni, C.; Kim, J.; et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci. 2018, 21, 1209–1217. [Google Scholar] [CrossRef]
- Pulous, F.E.; Cruz-Hernandez, J.C.; Yang, C.; Kaya, Z.; Paccalet, A.; Wojtkiewicz, G.; Capen, D.; Brown, D.; Wu, J.W.; Schloss, M.J.; et al. Cerebrospinal fluid can exit into the skull bone marrow and instruct cranial hematopoiesis in mice with bacterial meningitis. Nat. Neurosci. 2022, 25, 567–576. [Google Scholar] [CrossRef]
- Staals, J.; Makin, S.D.J.; Doubal, F.N.; Dennis, M.S.; Wardlaw, J.M. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014, 83, 1228–1234. [Google Scholar] [CrossRef]
- Kwon, H.; Lynn, M.J.; Turan, T.N.; Derdeyn, C.P.; Fiorella, D.; Lane, B.F.; Montgomery, J.; Janis, L.S.; Rumboldt, Z.; Chimowitz, M.I. Frequency, Risk Factors, and Outcome of Coexistent Small Vessel Disease and Intracranial Arterial Stenosis. JAMA Neurol. 2016, 73, 36. [Google Scholar] [CrossRef] [PubMed]
- Das, A.S.; Regenhardt, R.W.; Vernooij, M.W.; Blacker, D.; Charidimou, A.; Viswanathan, A. Asymptomatic Cerebral Small Vessel Disease: Insights from Population-Based Studies. J. Stroke 2019, 21, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K.N.; Sanggaard, S.; Mestre, H.; Lee, H.; Kostrikov, S.; Xavier, A.L.R.; Gjedde, A.; Benveniste, H.; Nedergaard, M. Impaired Glymphatic Transport in Spontaneously Hypertensive Rats. J. Neurosci. 2019, 39, 6365–6377. [Google Scholar] [CrossRef]
- Mestre, H.; Tithof, J.; Du, T.; Song, W.; Peng, W.; Sweeney, A.M.; Olveda, G.; Thomas, J.H.; Nedergaard, M.; Kelley, D.H. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 2018, 9, 4878. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rutten-Jacobs, L.; Liu, M.; Markus, H.S.; Traylor, M. Causal Impact of Type 2 Diabetes Mellitus on Cerebral Small Vessel Disease. Stroke 2018, 49, 1325–1331. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Ding, G.; Davoodi-Bojd, E.; Li, Q.; Li, L.; Sadry, N.; Nedergaard, M.; Chopp, M.; Zhang, Z. Impairment of the glymphatic system after diabetes. J. Cereb. Blood Flow Metab. 2017, 37, 1326–1337. [Google Scholar] [CrossRef]
- Yang, G.; Deng, N.; Liu, Y.; Gu, Y.; Yao, X. Evaluation of Glymphatic System Using Diffusion MR Technique in T2DM Cases. Front. Hum. Neurosci. 2020, 14, 300. [Google Scholar] [CrossRef]
- Pfrieger, F.W.; Ungerer, N. Cholesterol metabolism in neurons and astrocytes. Prog. Lipid Res. 2011, 50, 357–371. [Google Scholar] [CrossRef]
- Kim, J.S. Role of Blood Lipid Levels and Lipid-Lowering Therapy in Stroke Patients with Different Levels of Cerebral Artery Diseases: Reconsidering Recent Stroke Guidelines. J. Stroke 2021, 23, 149–161. [Google Scholar] [CrossRef]
- Belloy, M.E.; Napolioni, V.; Greicius, M.D. A Quarter Century of APOE and Alzheimer’s Disease: Progress to Date and the Path Forward. Neuron 2019, 101, 820–838. [Google Scholar] [CrossRef] [PubMed]
- Pankiewicz, J.E.; Lizinczyk, A.M.; Franco, L.A.; Diaz, J.R.; Marta-Ariza, M.; Sadowski, M.J. Absence of Apolipoprotein E is associated with exacerbation of prion pathology and promotes microglial neurodegenerative phenotype. Acta Neuropathol. Commun. 2021, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Achariyar, T.M.; Li, B.; Peng, W.; Verghese, P.B.; Shi, Y.; McConnell, E.; Benraiss, A.; Kasper, T.; Song, W.; Takano, T.; et al. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol. Neurodegener. 2016, 11, 74. [Google Scholar] [CrossRef]
- Mentis, A.A.; Dardiotis, E.; Chrousos, G.P. Apolipoprotein E4 and meningeal lymphatics in Alzheimer disease: A conceptual framework. Mol. Psychiatry 2021, 26, 1075–1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiong, M.; Gratuze, M.; Bao, X.; Shi, Y.; Andhey, P.S.; Manis, M.; Schroeder, C.; Yin, Z.; Madore, C.; et al. Selective removal of astrocytic APOE4 strongly protects against tau-mediated neurodegeneration and decreases synaptic phagocytosis by microglia. Neuron 2021, 109, 1657–1674. [Google Scholar] [CrossRef]
- Mahan, T.E.; Wang, C.; Bao, X.; Choudhury, A.; Ulrich, J.D.; Holtzman, D.M. Selective reduction of astrocyte apoE3 and apoE4 strongly reduces Abeta accumulation and plaque-related pathology in a mouse model of amyloidosis. Mol. Neurodegener. 2022, 17, 13. [Google Scholar] [CrossRef]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The neurovascular unit—Concept review. Acta Physiol. 2014, 210, 790–798. [Google Scholar] [CrossRef]
- Evans, L.E.; Taylor, J.L.; Smith, C.J.; Pritchard, H.A.T.; Greenstein, A.S.; Allan, S.M. Cardiovascular comorbidities, inflammation, and cerebral small vessel disease. Cardiovasc. Res. 2021, 117, 2575–2588. [Google Scholar] [CrossRef]
- Sabayan, B.; Westendorp, R.G.J. Neurovascular-glymphatic dysfunction and white matter lesions. Geroscience 2021, 43, 1635–1642. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Vinitsky, H.S.; Sun, Q.; Stæger, F.F.; Sigurdsson, B.; Mortensen, K.N.; Lilius, T.O.; Nedergaard, M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019, 5, v5447. [Google Scholar] [CrossRef]
- Dreha-Kulaczewski, S.; Joseph, A.A.; Merboldt, K.D.; Ludwig, H.C.; Gartner, J.; Frahm, J. Inspiration is the major regulator of human CSF flow. J. Neurosci. 2015, 35, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, S.M.; Schenk, S.; Leichliter, A.; Leibson, Z.; Fukamachi, K.; Luciano, M.G. Chronic hydrocephalus-induced changes in cerebral blood flow: Mediation through cardiac effects. J. Cereb. Blood Flow Metab. 2006, 26, 1298–1310. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a20412. [Google Scholar] [CrossRef] [PubMed]
- Burgmans, S.; van de Haar, H.J.; Verhey, F.R.; Backes, W.H. Amyloid-beta interacts with blood-brain barrier function in dementia: A systematic review. J. Alzheimer’s Dis. 2013, 35, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Verheggen, I.C.M.; Van Boxtel, M.P.J.; Verhey, F.R.J.; Jansen, J.F.A.; Backes, W.H. Interaction between blood-brain barrier and glymphatic system in solute clearance. Neurosci. Biobehav. Rev. 2018, 90, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Van Assema, D.M.; Lubberink, M.; Bauer, M.; van der Flier, W.M.; Schuit, R.C.; Windhorst, A.D.; Comans, E.F.; Hoetjes, N.J.; Tolboom, N.; Langer, O.; et al. Blood-brain barrier P-glycoprotein function in Alzheimer’s disease. Brain 2012, 135, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Zuroff, L.; Daley, D.; Black, K.L.; Koronyo-Hamaoui, M. Clearance of cerebral Abeta in Alzheimer’s disease: Reassessing the role of microglia and monocytes. Cell. Mol. Life Sci. 2017, 74, 2167–2201. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Zeppenfeld, D.M.; Venkataraman, A.; Plog, B.A.; Liao, Y.; Deane, R.; Nedergaard, M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 2013, 33, 18190–18199. [Google Scholar] [CrossRef]
- Iliff, J.J.; Nedergaard, M. Is there a cerebral lymphatic system? Stroke 2013, 44, S93–S95. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nelson, L.H. Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Dudvarski, S.N.; Teodorczyk, M.; Ploen, R.; Zipp, F.; Schmidt, M. Microglia-blood vessel interactions: A double-edged sword in brain pathologies. Acta Neuropathol. 2016, 131, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Low, A.; Mak, E.; Malpetti, M.; Passamonti, L.; Nicastro, N.; Stefaniak, J.D.; Savulich, G.; Chouliaras, L.; Su, L.; Rowe, J.B.; et al. In vivo neuroinflammation and cerebral small vessel disease in mild cognitive impairment and Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2020, 92, 45–52. [Google Scholar] [CrossRef]
- Farkas, E.; Donka, G.; de Vos, R.A.; Mihaly, A.; Bari, F.; Luiten, P.G. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol. 2004, 108, 57–64. [Google Scholar] [CrossRef]
- Jalal, F.Y.; Yang, Y.; Thompson, J.; Lopez, A.C.; Rosenberg, G.A. Myelin loss associated with neuroinflammation in hypertensive rats. Stroke 2012, 43, 1115–1122. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Papadopoulos, Z.; Dykstra, T.; Brase, L.; Farias, F.G.; Wall, M.; Jiang, H.; Kodira, C.D.; de Lima, K.A.; Herz, J.; et al. Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature 2021, 593, 255–260. [Google Scholar] [CrossRef]
- Da, M.S.; Herz, J.; Wall, M.; Dykstra, T.; de Lima, K.A.; Norris, G.T.; Dabhi, N.; Kennedy, T.; Baker, W.; Kipnis, J. Aging-associated deficit in CCR7 is linked to worsened glymphatic function, cognition, neuroinflammation, and beta-amyloid pathology. Sci. Adv. 2021, 7, eabe4601. [Google Scholar] [CrossRef]
- Graham, M.S.; Mellinghoff, I.K. Meningeal lymphatics prime tumor immunity in glioblastoma. Cancer Cell 2021, 39, 304–306. [Google Scholar] [CrossRef]
- Hu, X.; Deng, Q.; Ma, L.; Li, Q.; Chen, Y.; Liao, Y.; Zhou, F.; Zhang, C.; Shao, L.; Feng, J.; et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 2020, 30, 229–243. [Google Scholar] [CrossRef]
- Song, E.; Mao, T.; Dong, H.; Boisserand, L.S.B.; Antila, S.; Bosenberg, M.; Alitalo, K.; Thomas, J.; Iwasaki, A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 2020, 577, 689–694. [Google Scholar] [CrossRef]
- Louveau, A.; Herz, J.; Alme, M.N.; Salvador, A.F.; Dong, M.Q.; Viar, K.E.; Herod, S.G.; Knopp, J.; Setliff, J.C.; Lupi, A.L.; et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 2018, 21, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Zhang, C.; Jeong, J.; Lee, S.; McConnell, M.; Utsumi, T.; Iwakiri, Y. Enhanced Meningeal Lymphatic Drainage Ameliorates Neuroinflammation and Hepatic Encephalopathy in Cirrhotic Rats. Gastroenterology 2021, 160, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Haffner, C. Proteostasis in Cerebral Small Vessel Disease. Front. Neurosci. 2019, 13, 1142. [Google Scholar] [CrossRef]
- Young, K.Z.; Xu, G.; Keep, S.G.; Borjigin, J.; Wang, M.M. Overlapping Protein Accumulation Profiles of CADASIL and CAA. Am. J. Pathol. 2021, 191, 1871–1887. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Achariyar, T.M.; Li, B.; Liao, Y.; Mestre, H.; Hitomi, E.; Regan, S.; Kasper, T.; Peng, S.; Ding, F.; et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2016, 93, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, X.; Xia, D.; Liu, H.; Tian, H.; Fu, Y.; Chen, Y.; Qin, C.; Wang, J.; Xiang, Z.; et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat. Med. 2021, 27, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef]
- Duncombe, J.; Kitamura, A.; Hase, Y.; Ihara, M.; Kalaria, R.N.; Horsburgh, K. Chronic cerebral hypoperfusion: A key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin. Sci. 2017, 131, 2451–2468. [Google Scholar] [CrossRef]
- Arba, F.; Mair, G.; Carpenter, T.; Sakka, E.; Sandercock, P.; Lindley, R.I.; Inzitari, D.; Wardlaw, J.M. Cerebral White Matter Hypoperfusion Increases with Small-Vessel Disease Burden. Data From the Third International Stroke Trial. J. Stroke Cerebrovasc. Dis. 2017, 26, 1506–1513. [Google Scholar] [CrossRef]
- Wong, S.M.; Jansen, J.F.A.; Zhang, C.E.; Hoff, E.I.; Staals, J.; van Oostenbrugge, R.J.; Backes, W.H. Blood-brain barrier impairment and hypoperfusion are linked in cerebral small vessel disease. Neurology 2019, 92, e1669–e1677. [Google Scholar] [CrossRef]
- Shibata, M.; Ohtani, R.; Ihara, M.; Tomimoto, H. White Matter Lesions and Glial Activation in a Novel Mouse Model of Chronic Cerebral Hypoperfusion. Stroke 2004, 35, 2598–2603. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yao, D.; Li, R.; Guo, X.; Hao, J.; Xie, M.; Li, J.; Pan, D.; Luo, X.; Yu, Z.; et al. Digoxin Ameliorates Glymphatic Transport and Cognitive Impairment in a Mouse Model of Chronic Cerebral Hypoperfusion. Neurosci. Bull. 2021, 38, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Blair, G.W.; Thrippleton, M.J.; Shi, Y.; Hamilton, I.; Stringer, M.; Chappell, F.; Dickie, D.A.; Andrews, P.; Marshall, I.; Doubal, F.N.; et al. Intracranial hemodynamic relationships in patients with cerebral small vessel disease. Neurology 2020, 94, e2258–e2269. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Thrippleton, M.J.; Marshall, I.; Wardlaw, J.M. Intracranial pulsatility in patients with cerebral small vessel disease: A systematic review. Clin. Sci. 2018, 132, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Thrippleton, M.J.; Makin, S.D.; Marshall, I.; Geerlings, M.I.; de Craen, A.J.; van Buchem, M.A.; Wardlaw, J.M. Cerebral blood flow in small vessel disease: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2016, 36, 1653–1667. [Google Scholar] [CrossRef]
- Liu, Q.; Yan, L.; Huang, M.; Zeng, H.; Satyanarayanan, S.K.; Shi, Z.; Chen, D.; Lu, J.; Pei, Z.; Yao, X.; et al. Experimental alcoholism primes structural and functional impairment of the glymphatic pathway. Brain Behav. Immun. 2020, 85, 106–119. [Google Scholar] [CrossRef]
- Riba-Llena, I.; Jiménez-Balado, J.; Castañé, X.; Girona, A.; López-Rueda, A.; Mundet, X.; Jarca, C.I.; Álvarez-Sabin, J.; Montaner, J.; Delgado, P. Arterial Stiffness Is Associated With Basal Ganglia Enlarged Perivascular Spaces and Cerebral Small Vessel Disease Load. Stroke 2018, 49, 1279–1281. [Google Scholar] [CrossRef]
- Shi, Y.; Thrippleton, M.J.; Blair, G.W.; Dickie, D.A.; Marshall, I.; Hamilton, I.; Doubal, F.N.; Chappell, F.; Wardlaw, J.M. Small vessel disease is associated with altered cerebrovascular pulsatility but not resting cerebral blood flow. J. Cereb. Blood Flow Metab. 2020, 40, 85–99. [Google Scholar] [CrossRef]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Ao, D.; Zhang, D.; Zhai, F.; Zhang, J.; Han, F.; Li, M.; Ni, J.; Yao, M.; Zhang, S.; Cui, L.; et al. Brain deep medullary veins on 3-T MRI in a population-based cohort. J. Cereb. Blood Flow Metab. 2021, 41, 561–568. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, Y.; Zhang, W.; Li, Q.; Sun, J.; Lou, M. MRI-visible perivascular spaces in basal ganglia but not centrum semiovale or hippocampus were related to deep medullary veins changes. J. Cereb. Blood Flow Metab. 2022, 42, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Boulouis, G.; Gurol, M.E.; Ayata, C.; Bacskai, B.J.; Frosch, M.P.; Viswanathan, A.; Greenberg, S.M. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain 2017, 140, 1829–1850. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ahn, J.H.; Yang, H.; Lee, P.; Koh, G.Y.; Jeong, Y. Cerebral amyloid angiopathy aggravates perivascular clearance impairment in an Alzheimer’s disease mouse model. Acta Neuropathol. Com. 2020, 8, 181. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.; Koundal, S.; Elkin, R.; Zhu, X.; Monte, B.; Xu, F.; Dai, F.; Pedram, M.; Lee, H.; et al. Cerebral amyloid angiopathy is associated with glymphatic transport reduction and time-delayed solute drainage along the neck arteries. Nat. Aging 2022, 2, 214–223. [Google Scholar] [CrossRef]
- Li, M.; Kitamura, A.; Beverley, J.; Koudelka, J.; Duncombe, J.; Lennen, R.; Jansen, M.A.; Marshall, I.; Platt, B.; Wiegand, U.K.; et al. Impaired Glymphatic Function and Pulsation Alterations in a Mouse Model of Vascular Cognitive Impairment. Front. Aging Neurosci. 2022, 13, 788519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, R.; Ye, Y.; Wang, S.; Jiaerken, Y.; Hong, H.; Li, K.; Zeng, Q.; Luo, X.; Xu, X.; et al. The Influence of Demographics and Vascular Risk Factors on Glymphatic Function Measured by Diffusion Along Perivascular Space. Front. Aging Neurosci. 2021, 13, 693787. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ding, F.; Deng, S.; Guo, X.; Wang, W.; Iliff, J.J.; Nedergaard, M. Focal Solute Trapping and Global Glymphatic Pathway Impairment in a Murine Model of Multiple Microinfarcts. J. Neurosci. 2017, 37, 2870–2877. [Google Scholar] [CrossRef]
- Kim, H.W.; Hong, J.; Jeon, J.C. Cerebral Small Vessel Disease and Alzheimer’s Disease: A Review. Front. Neurol. 2020, 11, 927. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; He, W.; Tu, H.; Tang, Z.; Xiao, M.; Yan, L.J. Cerebral small vessel disease and Alzheimer’s disease. Clin. Interv. Aging 2015, 10, 1695–1704. [Google Scholar] [CrossRef]
- Harrison, I.F.; Ismail, O.; Machhada, A.; Colgan, N.; Ohene, Y.; Nahavandi, P.; Ahmed, Z.; Fisher, A.; Meftah, S.; Murray, T.K.; et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain 2020, 143, 2576–2593. [Google Scholar] [CrossRef]
- Xu, Z.; Xiao, N.; Chen, Y.; Huang, H.; Marshall, C.; Gao, J.; Cai, Z.; Wu, T.; Hu, G.; Xiao, M. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol. Neurodegener. 2015, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Postnov, D.; Penzel, T.; Kurths, J. Sleep as a Novel Biomarker and a Promising Therapeutic Target for Cerebral Small Vessel Disease: A Review Focusing on Alzheimer’s Disease and the Blood-Brain Barrier. Int. J. Mol. Sci. 2020, 21, 6293. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Zhao, M.; Chen, Y.; Yang, M.; Wang, Y. The Underlying Role of the Glymphatic System and Meningeal Lymphatic Vessels in Cerebral Small Vessel Disease. Biomolecules 2022, 12, 748. https://doi.org/10.3390/biom12060748
Tian Y, Zhao M, Chen Y, Yang M, Wang Y. The Underlying Role of the Glymphatic System and Meningeal Lymphatic Vessels in Cerebral Small Vessel Disease. Biomolecules. 2022; 12(6):748. https://doi.org/10.3390/biom12060748
Chicago/Turabian StyleTian, Yu, Mengxi Zhao, Yiyi Chen, Mo Yang, and Yilong Wang. 2022. "The Underlying Role of the Glymphatic System and Meningeal Lymphatic Vessels in Cerebral Small Vessel Disease" Biomolecules 12, no. 6: 748. https://doi.org/10.3390/biom12060748