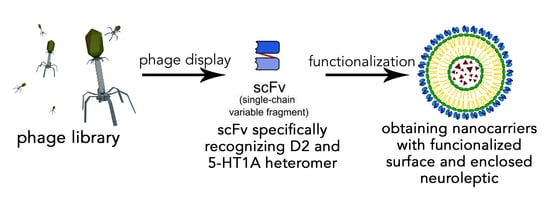
Dopamine D2 and Serotonin 5-HT1A Dimeric Receptor-Binding Monomeric Antibody scFv as a Potential Ligand for Carrying Drugs Targeting Selected Areas of the Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. scFv Production
2.2. Protein Stability
2.3. Cell Culture
2.4. Cytotoxicity Tests
2.5. Second Messengers
2.6. Cytokine Production
2.7. Nanogold Coupling
2.8. Transmission Electron Microscopy Imagining of the Nanogold Particles
2.9. ELISA
2.10. The Blood–Brain Barrier Model Transition
2.11. Animals and scFvD2–5-HT1A Antibody Administration
2.12. Locomotor Activity
2.13. Statistical Analysis
3. Results and Discussion
3.1. Protein Production
3.2. Protein Stability
3.3. Transmission Electron Microscopy of Nanogold Particles
3.4. Cytotoxicity
3.5. Cytokine Production
3.6. Second Messengers
3.7. ELISA
3.8. The Blood–Brain Barrier Model Transition
3.9. Locomotor Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosseini, M.; Haji-Fatahaliha, M.; Jadidi-Niaragh, F.; Majidi, J.; Yousefi, M. The use of nanoparticles as a promising therapeutic approach in cancer immunotherapy. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Kadam, R.S.; Bourne, D.W.A.; Kompella, U.B. Nano-Advantage in Enhanced Drug Delivery with Biodegradable Nanoparticles: Contribution of Reduced Clearance. Drug Metab. Dispos. 2012, 40, 1380–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risch, S.J. Encapsulation: Overview of Uses and Techniques; American Chemical Society: Washington, DC, USA, 1995; pp. 2–7. [Google Scholar] [CrossRef] [Green Version]
- Chavarri, M.; Maranon, I.; Carmen, M. Encapsulation Technology to Protect Probiotic Bacteria. In Probiotics; InTech: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Narang, A.S.; Delmarre, D.; Gao, D. Stable drug encapsulation in micelles and microemulsions. Int. J. Pharm. 2007, 345, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Maitra, A.N.; Patanjali, P.K.; Sharma, P. Hollow gold nanoparticles encapsulating horseradish peroxidase. Biomaterials 2005, 26, 6743–6753. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, W.M.; Kyriakides, T.R. Cell Interactions with Polymers, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Uludag, H.; De Vos, P.; Tresco, P.A. Technology of mammalian cell encapsulation. Adv. Drug Deliv. Rev. 2000, 42, 29–64. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [Green Version]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The Potential Advantages of Nanoparticle Drug Delivery Systems in Chemotherapy of Tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef] [Green Version]
- Marcotte, H.; Hammarström, L. Passive Immunization: Toward Magic Bullets, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 2. [Google Scholar] [CrossRef]
- Ahmad, Z.A.; Yeap, S.K.; Ali, A.M.; Ho, W.Y.; Alitheen, N.B.M.; Hamid, M. scFv Antibody: Principles and Clinical Application. Clin. Dev. Immunol. 2012, 2012, 980250. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Fic, E.; Bzowska, M.; Dziedzicka-Wasylewska, M. Isolation of Human Monoclonal scfv Antibody Specifically Recognizing the D2-5-Ht1a Heteromer. J. New Dev. Chem. 2019, 2, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Huang, C.C.; Huang, W.; Tang, W.H.; Rait, A.; Yin, Y.Z.; Cruz, I.; Xiang, L.M.; Pirollo, K.F.; Chang, E.H. Systemic Tumor-Targeted Gene Delivery by Anti-Transferrin Receptor ScFv-Immunoliposomes. Mol. Cancer Ther. 2002, 1, 337–346. [Google Scholar] [PubMed]
- Weisser, N.E.; Hall, J.C. Applications of single-chain variable fragment antibodies in therapeutics and diagnostics. Biotechnol. Adv. 2009, 27, 502–520. [Google Scholar] [CrossRef]
- Safdari, Y.; Ahmadzadeh, V.; Khalili, M.; Jaliani, H.Z.; Zarei, V.; Erfani-Moghadam, V. Use of Single-Chain Antibody Derivatives for Targeted Drug Delivery. Mol. Med. 2016, 22, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Monnier, P.P.; Vigouroux, R.J.; Tassew, N.G. In Vivo Applications of Single Chain Fv (Variable Domain) (scFv) Fragments. Antibodies 2013, 2, 193–208. [Google Scholar] [CrossRef]
- Güell-Bosch, J.; Lope-Piedrafita, S.; Esquerda-Canals, G.; Montoliu-Gaya, L.; Villegas, S. Progression of Alzheimer’s Disease and Effect of ScFv-H3D6 Immunotherapy in the 3xTg-AD Mouse Model: An in Vivo Longitudinal Study Using Magnetic Resonance Imaging and Spectroscopy. NMR Biomed. 2020, 33, e4263. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, G.M.; Chen, Y.Y.; Tian, Y.H.; Yue, Y.; Zhang, G.L. Expression, production, and renaturation of a functional single-chain variable antibody fragment (scFv) against human ICAM-1. Braz. J. Med Biol. Res. 2014, 47, 540–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Ji, Y.; Wu, X.; Xu, H. Trafficking of Gold Nanorods in Breast Cancer Cells: Uptake, Lysosome Maturation, and Elimination. ACS Appl. Mater. Interfaces 2013, 5, 9856–9865. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Mernaugh, R.L.; Zeng, X. Single chain fragment variable recombinant antibody functionalized gold nanoparticles for a highly sensitive colorimetric immunoassay. Biosens. Bioelectron. 2009, 24, 2853–2857. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.D.; Weaver-Feldhaus, J.; Gray, S.A.; Siegel, R.W.; Feldhaus, M.J. Production, purification, and characterization of human scFv antibodies expressed in Saccharomyces cerevisiae, Pichia pastoris, and Escherichia coli. Protein Expr. Purif. 2005, 42, 255–267. [Google Scholar] [CrossRef]
- Jefferis, R. Recombinant antibody therapeutics: The impact of glycosylation on mechanisms of action. Trends Pharmacol. Sci. 2009, 30, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Taura, F.; Tsuchihashi, R.; Putalun, W.; Kinjo, J.; Tanaka, H.; Morimoto, S. Expression, Purification, and Characterization of Anti-Plumbagin Single-Chain Variable Fragment Antibody in Sf9 Insect Cell. Hybridoma 2010, 29, 481–488. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhang, C.; Liu, Y.; Liu, X. Isolation of single chain variable fragment (scFv) specific for Cry1C toxin from human single fold scFv libraries. Toxicon 2012, 60, 1290–1297. [Google Scholar] [CrossRef]
- Frenzel, A.; Hust, M.; Schirrmann, T. Expression of Recombinant Antibodies. Front. Immunol. 2013, 4, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galeffi, P.; Lombardi, A.; Pietraforte, I.; Novelli, F.; Di Donato, M.; Sperandei, M.; Tornambé, A.; Fraioli, R.; Martayan, A.; Natali, P.G.; et al. Functional expression of a single-chain antibody to ErbB-2 in plants and cell-free systems. J. Transl. Med. 2006, 4, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ossysek, K.; Uchański, T.; Kulesza, M.; Bzowska, M.; Klaus, T.; Woś, K.; Madej, M.; Bereta, J. A new expression vector facilitating production and functional analysis of scFv antibody fragments selected from Tomlinson I + J phagemid libraries. Immunol. Lett. 2015, 167, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRX 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Vykhodtseva, N.; McDannold, N.; Hynynen, K. Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. Ultrasonics 2008, 48, 279–296. [Google Scholar] [CrossRef] [Green Version]
- Boado, R.J.; Zhang, Y.; Zhang, Y.; Xia, C.-F.; Wang, Y.; Pardridge, W.M. Genetic engineering of a lysosomal enzyme fusion protein for targeted delivery across the human blood-brain barrier. Biotechnol. Bioeng. 2008, 99, 475–484. [Google Scholar] [CrossRef]
- Boado, R.J.; Zhang, Y.; Zhang, Y.; Pardridge, W.M. Humanization of anti-human insulin receptor antibody for drug targeting across the human blood–brain barrier. Biotechnol. Bioeng. 2007, 96, 381–391. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.D. Review and management of clozapine side effects. J. Clin. Psychiatry 2000, 61, 14–17. [Google Scholar] [PubMed]
- Bardin, L.; Auclair, A.; Kleven, M.S.; Prinssen, E.P.; Koek, W.; Newman-Tancredi, A.; Depoortère, R. Pharmacological profiles in rats of novel antipsychotics with combined dopamine D2/serotonin 5-HT1A activity: Comparison with typical and atypical conventional antipsychotics. Behav. Pharmacol. 2007, 18, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Etievant, A.; Bétry, C.; Haddjeri, N. Partial Dopamine D2/Serotonin 5-HT1A Receptor Agonists as New Therapeutic Agents. Open Neuropsychopharmacol. J. 2010, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Szafran, K.; Faron-Górecka, A.; Kolasa, M.; Kuśmider, M.; Solich, J.; Zurawek, D.; Dziedzicka-Wasylewska, M. Potential role of G protein-coupled receptor (GPCR) heterodimerization in neuropsychiatric disorders: A focus on depression. Pharmacol. Rep. 2013, 65, 1498–1505. [Google Scholar] [CrossRef]
- Szlachta, M.; Kuśmider, M.; Pabian, P.; Solich, J.; Kolasa, M.; Żurawek, D.; Dziedzicka-Wasylewska, M.; Faron-Górecka, A. Repeated Clozapine Increases the Level of Serotonin 5-HT1AR Heterodimerization with 5-HT2A or Dopamine D2 Receptors in the Mouse Cortex. Front. Mol. Neurosci. 2018, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Faron-Górecka, A.; Szlachta, M.; Kolasa, M.; Solich, J.; Górecki, A.; Kuśmider, M.; Żurawek, D.; Dziedzicka-Wasylewska, M. Understanding GPCR dimerization. Methods Cell Biol. 2019, 149, 155–178. [Google Scholar] [CrossRef]
- Łukasiewicz, S. Different Strategies used in the Production of human monoclonal scfv Antibodies Specific to Dimers of Membrane Receptors. J. Biol. Med. 2017, 3, 014–020. [Google Scholar] [CrossRef] [Green Version]
- Łukasiewicz, S.; Stachowicz, A.; Bzowska, M.; Dziedzicka-Wasylewska, M. Comparison of Various Selection Strategies Used for Isolation of Human Monoclonal scFv Antibody Specific to GPCRs Heteromers. J. Pharmacol. Pharm. Res. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Imperatore, R.; Carotenuto, G.; Di Grazia, M.A.; Ferrandino, I.; Palomba, L.; Mariotti, R.; Vitale, E.; De Nicola, S.; Longo, A.; Cristino, L. Imidazole-stabilized gold nanoparticles induce neuronal apoptosis: Anin vitroandin vivostudy. J. Biomed. Mater. Res. Part A 2015, 103, 1436–1446. [Google Scholar] [CrossRef]
- Fatima, N.; Gromnicova, R.; Loughlin, A.; Sharrack, B.; Male, D. Gold nanocarriers for transport of oligonucleotides across brain endothelial cells. PLoS ONE 2020, 15, e0236611. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Perez-Iratxeta, C.; Andrade-Navarro, M.A. K2D2: Estimation of protein secondary structure from circular dichroism spectra. BMC Struct. Biol. 2008, 8, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis-Jeune, C.; Andrade-Navarro, M.A.; Perez-Iratxeta, C. Prediction of protein secondary structure from circular dichroism using theoretically derived spectra. Proteins Struct. Funct. Bioinform. 2012, 80, 374–381. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.-H.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micsonai, A.; Wien, F.; Bulyáki, É.; Kun, J.; Moussong, É.; Lee, Y.-H.; Goto, Y.; Réfrégiers, M.; Kardos, J. BeStSel: A web server for accurate protein secondary structure prediction and fold recognition from the circular dichroism spectra. Nucleic Acids Res. 2018, 46, W315–W322. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Błasiak, E.; Szczepanowicz, K.; Guzik, K.; Bzowska, M.; Warszyński, P.; Dziedzicka-Wasylewska, M. The interaction of clozapine loaded nanocapsules with the hCMEC/D3 cells—In vitro model of blood brain barrier. Colloids Surf. B Biointerfaces 2017, 159, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Expedeon. InnovaCoat GOLD (20 OD) Conjugation Kit Manual; Innova Biosciences Ltd.: Cambridge, UK, 2018. [Google Scholar]
- Baneyx, F. Recombinant protein expression in Escherichia coli. Curr. Opin. Biotechnol. 1999, 10, 411–421. [Google Scholar] [CrossRef]
- Baneyx, F.; Mujacic, M. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 2004, 22, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Walls, D.; Walker, J.M. Protein Chromatography. Protein Chromatogr. 2017, 1485, 423. [Google Scholar] [CrossRef]
- Vermeer, A.W.P.; Norde, W. The Thermal Stability of Immunoglobulin: Unfolding and Aggregation of a Multi-Domain Protein. Biophys. J. 2000, 78, 394–404. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Sereno, A.J.; Huang, F.; Lewis, S.M.; Lieu, R.L.; Weldon, C.; Torres, C.; Fine, C.; Batt, M.A.; Fitchett, J.R.; et al. Fab-based bispecific antibody formats with robust biophysical properties and biological activity. mAbs 2015, 7, 470–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Park, H.; Kim, M.; Seo, Y.; Lee, Y.; Byun, S.J.; Lee, S.; Kwon, M.-H. Functional stability of 3D8 scFv, a nucleic acid-hydrolyzing single chain antibody, under different biochemical and physical conditions. Int. J. Pharm. 2015, 496, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoliu-Gaya, L.; Murciano-Calles, J.; Martinez, J.C.; Villegas, S. Towards the improvement in stability of an anti-Aβ single-chain variable fragment, scFv-h3D6, as a way to enhance its therapeutic potential. Amyloid 2017, 24, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Rathore, A.S.; Gupta, R.D. Evaluation of scFv protein recovery from E. coli by in vitro refolding and mild solubilization process. Microb. Cell Factories 2019, 18, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Shafer, T.J.; Atchison, W.D. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: A model for neurotoxicological studies. NeuroToxicology 1991, 12, 473–492. [Google Scholar]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- Somensi, N.; Rabelo, T.K.; Guimarães, A.G.; Quintans-Junior, L.J.; de Souza Araújo, A.A.; Moreira, J.C.F.; Gelain, D.P. Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW 264.7 macrophages through ERK1/2 and NF-kB pathway. Int. Immunopharmacol. 2019, 75, 105743. [Google Scholar] [CrossRef]
- Cheng, L.; Ren, Y.; Lin, D.; Peng, S.; Zhong, B.; Ma, Z. The Anti-Inflammatory Properties of Citrus wilsonii Tanaka Extract in LPS-Induced RAW 264.7 and Primary Mouse Bone Marrow-Derived Dendritic Cells. Molecules 2017, 22, 1213. [Google Scholar] [CrossRef]
- Bigl, K.; Schmitt, A.; Meiners, I.; Münch, G.; Arendt, T. Comparison of results of the CellTiter Blue, the tetrazolium (3-[4,5-dimethylthioazol-2-yl]-2,5-diphenyl tetrazolium bromide), and the lactate dehydrogenase assay applied in brain cells after exposure to advanced glycation endproducts. Toxicol. Vitr. 2007, 21, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, W.; Jiang, X.; Bai, S.; Liu, J.; Liu, X.; Shi, Y.; Kuai, Z.; Kong, W.; Gao, R.; et al. Using near-infrared enhanced thermozyme and scFv dual-conjugated Au nanorods for detection and targeted photothermal treatment of Alzheimer’s disease. Theranostics 2019, 9, 2268–2281. [Google Scholar] [CrossRef] [PubMed]
- Behring, S.; Hänsch, R.; Helmsing, S.; Schirrmann, T.; Schubert, M. Screening for scFv-fragments that are stable and active in the cytosol. Hum. Antibodies 2020, 28, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska-Wedzina, A.; Chodaczek, G.; Chudzian, J.; Borek, A.; Zakrzewska, M.; Otlewski, J. High-Affinity Internalizing Human scFv-Fc Antibody for Targeting FGFR1-Overexpressing Lung Cancer. Mol. Cancer Res. 2017, 15, 1040–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, C. Preface. PCR Primer Design. Methods Mol. Biol. 2015, 1275, vii. [Google Scholar] [CrossRef] [Green Version]
- Hofstetter, A.R.; Eberle, K.C.; Venn-Watson, S.K.; Jensen, E.D.; Porter, T.J.; Waters, T.E.; Sacco, R.E. Monitoring bottlenose dolphin leukocyte cytokine mRNA responsiveness by qPCR. PLoS ONE 2017, 12, e0189437. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028456. [Google Scholar] [CrossRef]
- Horiuchi, T.; Mitoma, H.; Harashima, S.-I.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-α: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef] [Green Version]
- Anavi, S.; Tirosh, O. iNOS as a metabolic enzyme under stress conditions. Free Radic. Biol. Med. 2020, 146, 16–35. [Google Scholar] [CrossRef]
- Ahmad, N.; Ansari, M.Y.; Haqqi, T.M. Role of iNOS in osteoarthritis: Pathological and therapeutic aspects. J. Cell. Physiol. 2020, 235, 6366–6376. [Google Scholar] [CrossRef] [PubMed]
- Dąbek, J.; Kułach, A.; Gąsior, Z. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB): A new potential therapeutic target in atherosclerosis? Pharmacol. Rep. 2010, 62, 778–783. [Google Scholar] [CrossRef]
- Schmitz, M.L.; Bacher, S.; Kracht, M. IκB-independent control of NF-κB activity by modulatory phosphorylations. Trends Biochem. Sci. 2001, 26, 186–190. [Google Scholar] [CrossRef]
- Hay, R.T.; Vuillard, L.; Desterro, J.M.P.; Rodriguez, M.S. Control of NF-κB Transcriptional Activation by Signal Induced Proteolysis of IκBα. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 1601–1609. [Google Scholar] [CrossRef]
- Mulero, M.C.; Huxford, T.; Ghosh, G. NF-κ B, I κ B, and IKK: Integral Signaling; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Stoiber, S.; Cadilha, B.L.; Benmebarek, M.-R.; Lesch, S.; Endres, S.; Kobold, S. Limitations in the Design of Chimeric Antigen Receptors for Cancer Therapy. Cells 2019, 8, 472. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Saraswat, D.; Tati, S.; Edgerton, M. Novel Aggregation Properties of Candida albicans Secreted Aspartyl Proteinase Sap6 Mediate Virulence in Oral Candidiasis. Infect. Immun. 2015, 83, 2614–2626. [Google Scholar] [CrossRef] [Green Version]
- Zajc, C.U.; Salzer, B.; Taft, J.M.; Reddy, S.T.; Lehner, M.; Traxlmayr, M.W. Driving CARs with alternative navigation tools—The potential of engineered binding scaffolds. FEBS J. 2021, 288, 2103–2118. [Google Scholar] [CrossRef]
- van Rijn, R.; Whistler, J.L.; Waldhoer, M. Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr. Opin. Pharmacol. 2010, 10, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Hasbi, A.; Sivasubramanian, M.; Milenkovic, M.; Komarek, K.; Madras, B.K.; George, S.R. Dopamine D1-D2 receptor heteromer expression in key brain regions of rat and higher species: Upregulation in rat striatum after cocaine administration. Neurobiol. Dis. 2020, 143, 105017. [Google Scholar] [CrossRef]
- Kolasa, M.; Solich, J.; Faron-Górecka, A.; Żurawek, D.; Pabian, P.; Łukasiewicz, S.; Kuśmider, M.; Szafran-Pilch, K.; Szlachta, M.; Dziedzicka-Wasylewska, M. Paroxetine and Low-dose Risperidone Induce Serotonin 5-HT1A and Dopamine D2 Receptor Heteromerization in the Mouse Prefrontal Cortex. Neuroscience 2018, 377, 184–196. [Google Scholar] [CrossRef]
- Johnstone, E.K.M.; Pfleger, K.D.G. Receptor-Heteromer Investigation Technology and its application using BRET. Front. Endocrinol. 2012, 3, 101. [Google Scholar] [CrossRef] [Green Version]
- Łukasiewicz, S.; Błasiak, E.; Szafran-Pilch, K.; Dziedzicka-Wasylewska, M. Dopamine D2 and serotonin 5-HT1A receptor interaction in the context of the effects of antipsychotics—In vitro studies. J. Neurochem. 2016, 137, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold nanoparticles: Distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, e3702518. [Google Scholar] [CrossRef]
- Jones, A.R.; Stutz, C.C.; Zhou, Y.; Marks, J.D.; Shusta, E.V. Identifying blood-brain-barrier selective single-chain antibody fragments. Biotechnol. J. 2014, 9, 664–674. [Google Scholar] [CrossRef] [Green Version]
- Weksler, B.; Romero, I.A.; Couraud, P.-O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Eigenmann, D.E.; Xue, G.; Kim, K.S.; Moses, A.V.; Hamburger, M.; Oufir, M. Comparative Study of Four Immortalized Human Brain Capillary Endothelial Cell Lines, HCMEC/D3, HBMEC, TY10, and BB19, and Optimization of Culture Conditions, for an In Vitro Blood-Brain Barrier Model for Drug Permeability Studies Fluids and Barriers of TH; BioMed Central: London, UK, 2013; Volume 10. [Google Scholar]
- Biemans, E.A.L.M.; Jäkel, L.; de Waal, R.M.W.; Kuiperij, H.B.; Verbeek, M.M. Limitations of the hCMEC/D3 cell line as a model for Aβ clearance by the human blood-brain barrier. J. Neurosci. Res. 2017, 95, 1513–1522. [Google Scholar] [CrossRef]
- Obermeier, B.; Verma, A.; Ransohoff, R.M. The Blood-Brain Barrier, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 133. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Szczepanowicz, K.; Podgórna, K.; Błasiak, E.; Majeed, N.; Ogren, S.O.Ö.; Nowak, W.; Warszyński, P.; Dziedzicka-Wasylewska, M. Encapsulation of clozapine in polymeric nanocapsules and its biological effects. Colloids Surf. B Biointerfaces 2016, 140, 342–352. [Google Scholar] [CrossRef] [PubMed]










| Gene | Starter Forward (5′→3′) | Starter Reverse (5′→3′) |
|---|---|---|
| GAPDH | TCAACGGCACAGTCAAGG | ACTCCACGACATACTCAGC |
| IL-6 | TTCTCTGGGAAATCGTGGAAA | TCAGAATTGCCATTGCACAAC |
| TNF | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| iNO | TCCTACACCACACCAAAC | CTCCAATCTCTGCCTATCC |
| iκB | CTTGGTGACTTTGGGTGCTGAT | GCGAAACCAGGTCAGGATTC |
| Structural Motif | K2D2 and K2D3 | BeStSel |
|---|---|---|
| Alpha helix | 1.12% | 1.70% |
| Beta sheet | 36.30% | 38.00% |
| Unstructured/other | 61.95% | 60.30% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalik, A.; Majerek, M.; Mrowiec, K.; Solich, J.; Faron-Górecka, A.; Woźnicka, O.; Dziedzicka-Wasylewska, M.; Łukasiewicz, S. Dopamine D2 and Serotonin 5-HT1A Dimeric Receptor-Binding Monomeric Antibody scFv as a Potential Ligand for Carrying Drugs Targeting Selected Areas of the Brain. Biomolecules 2022, 12, 749. https://doi.org/10.3390/biom12060749
Kowalik A, Majerek M, Mrowiec K, Solich J, Faron-Górecka A, Woźnicka O, Dziedzicka-Wasylewska M, Łukasiewicz S. Dopamine D2 and Serotonin 5-HT1A Dimeric Receptor-Binding Monomeric Antibody scFv as a Potential Ligand for Carrying Drugs Targeting Selected Areas of the Brain. Biomolecules. 2022; 12(6):749. https://doi.org/10.3390/biom12060749
Chicago/Turabian StyleKowalik, Agata, Mateusz Majerek, Krzysztof Mrowiec, Joanna Solich, Agata Faron-Górecka, Olga Woźnicka, Marta Dziedzicka-Wasylewska, and Sylwia Łukasiewicz. 2022. "Dopamine D2 and Serotonin 5-HT1A Dimeric Receptor-Binding Monomeric Antibody scFv as a Potential Ligand for Carrying Drugs Targeting Selected Areas of the Brain" Biomolecules 12, no. 6: 749. https://doi.org/10.3390/biom12060749