Co-Occurrence of Interleukin-6 Receptor Asp358Ala Variant and High Plasma Levels of IL-6: An Evidence of IL-6 Trans-Signaling Activation in Deep Vein Thrombosis (DVT) Patients
Abstract
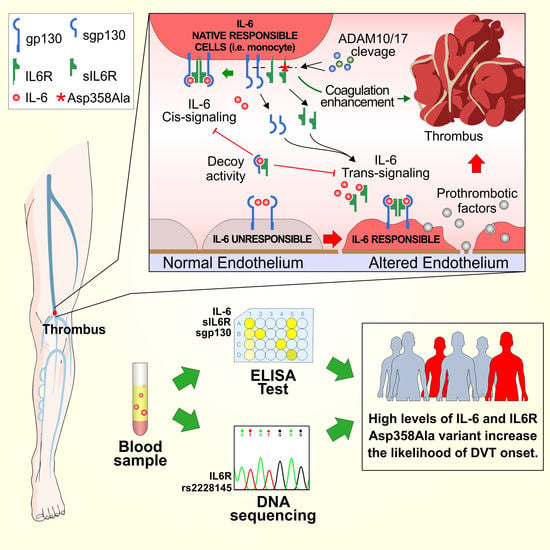
:1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Controls
2.2. Sample Collection and ELISA Test
2.3. IL6R Exon 9 Sequencing
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Patients and Healthy Controls
3.2. Plasma Levels of IL-6, sIL-6R, and sgp130
3.3. Association Analysis of IL-6 Plasma Levels and IL6R Polymorphisms in DVT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Scheller, J.; Rose-John, S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J. Clin. Investig. 2011, 121, 3375–3383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohleder, N.; Aringer, M.; Boentert, M. Role of interleukin-6 in stress, sleep, and fatigue. Ann. N. Y. Acad. Sci. 2012, 1261, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Elewaut, D.; McInnes, I.B.; Dayer, J.M.; Neurath, M.F. How cytokine networks fuel inflammation: Toward a cytokine-based disease taxonomy. Nat. Med. 2013, 19, 822–824. [Google Scholar] [CrossRef] [PubMed]
- Hodes, G.E.; Pfau, M.L.; Leboeuf, M.; Golden, S.A.; Christoffel, D.J.; Bregman, D.; Rebusi, N.; Heshmati, M.; Aleyasin, H.; Warren, B.L.; et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. USA 2014, 111, 16136–16141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bethin, K.E.; Vogt, S.K.; Muglia, L.J. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc. Natl. Acad. Sci. USA 2000, 97, 9317–9322. [Google Scholar] [CrossRef] [Green Version]
- Kraakman, M.J.; Kammoun, H.L.; Allen, T.L.; Deswaerte, V.; Henstridge, D.C.; Estevez, E.; Matthews, V.B.; Neill, B.; White, D.A.; Murphy, A.J.; et al. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell. Metab. 2015, 21, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Hirano, T. Revisiting the 1986 molecular cloning of interleukin 6. Front. Immunol. 2014, 5, 456. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, K.; Hirano, T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002, 13, 357–368. [Google Scholar] [CrossRef]
- Bajocchi, G.L.; Pipitone, N.; Boiardi, P.L.; Salvarani, C. Primary pathologic role of interleukin-6 in rheumatoid arthritis. Ital. J. Med. 2013, 2, 40–46. [Google Scholar] [CrossRef]
- Pola, R.; Gaetani, E.; Flex, A.; Aloi, F.; Papaleo, P.; Gerardino, L.; De Martini, D.; Flore, R.; Pola, P.; Bernabei, R. -174 G/C interleukin-6 gene polymorphism and increased risk of multi-infarct dementia: A case-control study. Exp. Gerontol. 2002, 37, 949–955. [Google Scholar] [CrossRef]
- Sarwar, N.; Butterworth, A.S.; Freitag, D.F.; Gregson, J.; Willeit, P.; Gorman, D.N.; Gao, P.; Saleheen, D.; Rendon, A.; Nelson, C.P.; et al. Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet 2012, 379, 1205–1213. [Google Scholar] [PubMed] [Green Version]
- Zhang, Y.; Zhang, Z.; Wei, R.; Miao, X.; Sun, S.; Liang, G.; Chu, C.; Zhao, L.; Zhu, X.; Guo, Q.; et al. IL (Interleukin)-6 Contributes to Deep Vein Thrombosis and Is Negatively Regulated by miR-338-5p. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, K.; Biswas, A.; Ranjan, R.; Kishor, K.; Pandey, H.; Kumar, R.; Mahapatra, M.; Oldenburg, J.; Saxena, R. Impact of interleukin 6 promoter polymorphisms (-174 G > C, -572 G > C and -597 G > A) on plasma IL-6 levels and their influence on the development of DVT: A study from India. Hematology 2018, 23, 833–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhter, M.S.; Biswas, A.; Abdullah, S.M.; Hobani, Y.; Ranjan, R.; Behari, M.; Saxena, R. Influence of Interleukin-6 (IL-6) Promoter Gene Polymorphisms (-174G>C, -572G>C, and -597G>A) on IL-6 Plasma Levels and Their Impact in the Development of Acute Ischemic Stroke in Young Indians. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619854136. [Google Scholar] [CrossRef] [Green Version]
- Vormittag, R.; Hsieh, K.; Kaider, A.; Minar, E.; Bialonczyk, C.; Hirschl, M.; Mannhalter, C.; Pabinger, I. Interleukin-6 and interleukin-6 promoter polymorphism (-174) G>C in patients with spontaneous venous thromboembolism. Thromb. Haemost. 2006, 95, 802–806. [Google Scholar] [CrossRef]
- Garbers, C.; Monhasery, N.; Aparicio-Siegmund, S.; Lokau, J.; Baran, P.; Nowell, M.A.; Jones, S.A.; Rose-John, S.; Scheller, J. The interleukin-6 receptor Asp358Ala single nucleotide polymorphism rs2228145 confers increased proteolytic conversion rates by ADAM proteases. Biochim. Biophys. Acta 2014, 1842, 1485–1494. [Google Scholar] [CrossRef] [Green Version]
- Salemi, R.; Tomasello, B.M.R.; Gattuso, G.; Signorelli, S.S.; Candido, S. Overactivation of IL6 cis-signaling in leukocytes is an inflammatory hallmark of deep vein thrombosis. Mol. Med. Rep. 2022, 25, 136. [Google Scholar] [CrossRef]
- Galicia, J.C.; Tai, H.; Komatsu, Y.; Shimada, Y.; Akazawa, K.; Yoshie, H. Polymorphisms in the IL-6 receptor (IL-6R) gene: Strong evidence that serum levels of soluble IL-6R are genetically influenced. Genes Immun. 2004, 5, 513–516. [Google Scholar] [CrossRef] [Green Version]
- Rafiq, S.; Frayling, T.M.; Murray, A.; Hurst, A.; Stevens, K.; Weedon, M.N.; Henley, W.; Ferrucci, L.; Bandinelli, S.; Corsi, A.M.; et al. A common variant of the interleukin 6 receptor (IL-6r) gene increases IL-6r and IL-6 levels, without other inflammatory effects. Genes Immun. 2007, 8, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Candido, S.; Tomasello, B.M.R.; Lavoro, A.; Falzone, L.; Gattuso, G.; Libra, M. Novel Insights into Epigenetic Regulation of IL6 Pathway: In Silico Perspective on Inflammation and Cancer Relationship. Int. J. Mol. Sci. 2021, 22, 10172. [Google Scholar] [CrossRef] [PubMed]
- Jostock, T.; Müllberg, J.; Ozbek, S.; Atreya, R.; Blinn, G.; Voltz, N.; Fischer, M.; Neurath, M.F.; Rose-John, S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem. 2001, 268, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Nowell, M.A.; Williams, A.S.; Carty, S.A.; Scheller, J.; Hayes, A.J.; Jones, G.W.; Richards, P.J.; Slinn, S.; Ernst, M.; Jenkins, B.J.; et al. Therapeutic targeting of IL-6 trans signaling counteracts STAT3 control of experimental inflammatory arthritis. J. Immunol. 2009, 182, 613–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Aken, B.E.; den Heijer, M.; Bos, G.M.; van Deventer, S.J.; Reitsma, P.H. Recurrent venous thrombosis and markers of inflammation. Thromb. Haemost. 2000, 83, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Van Aken, B.E.; Reitsma, P.H.; Rosendaal, F.R. Interleukin 8 and venous thrombosis: Evidence for a role of inflammation in thrombosis. Br. J. Haematol. 2002, 116, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, A.; Hafizi, S.; Rezaei, N. Inflammation in venous thromboembolism: Cause or consequence? Int. Immunopharmacol. 2015, 28, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, P.H.; Rosendaal, F.R. Activation of innate immunity in patients with venous thrombosis: The Leiden Thrombophilia Study. J. Thromb. Haemost. 2004, 2, 619–622. [Google Scholar] [CrossRef] [Green Version]
- Branchford, B.R.; Carpenter, S.L. The Role of Inflammation in Venous Thromboembolism. Front. Pediatr. 2018, 6, 142. [Google Scholar] [CrossRef]
- Tanaka, Y.; Martin Mola, E. IL-6 targeting compared to TNF targeting in rheumatoid arthritis: Studies of olokizumab, sarilumab and sirukumab. Ann. Rheum. Dis. 2014, 73, 1595–1597. [Google Scholar] [CrossRef] [Green Version]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Candido, S.; Lumera, G.; Barcellona, G.; Vetri, D.; Tumino, E.; Platania, I.; Frazzetto, E.; Privitera, G.; Incognito, C.; Gaudio, A.; et al. Direct oral anticoagulant treatment of deep vein thrombosis reduces IL-6 expression in peripheral mono-nuclear blood cells. Exp. Ther. Med. 2020, 20, 237. [Google Scholar] [CrossRef] [PubMed]
- Roumen-Klappe, E.M.; den Heijer, M.; van Uum, S.H.; van der Ven-Jongekrijg, J.; van der Graaf, F.; Wollersheim, H. Inflammatory response in the acute phase of deep vein thrombosis. J. Vasc. Surg. 2002, 35, 701–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downing, L.J.; Strieter, R.M.; Kadell, A.M.; Wilke, C.A.; Greenfield, L.J.; Wakefield, T.W. Low-dose low-molecular-weight heparin is anti-inflammatory during venous thrombosis. J. Vasc. Surg. 1998, 28, 848–854. [Google Scholar] [CrossRef] [Green Version]
- Reich, D.; Patterson, N.; Ramesh, V.; De Jager, P.L.; McDonald, G.J.; Tandon, A.; Choy, E.; Hu, D.; Tamraz, B.; Pawlikowska, L.; et al. Admixture mapping of an allele affecting interleukin 6 soluble receptor and interleukin 6 levels. Am. J. Hum. Genet. 2007, 80, 716–726. [Google Scholar] [CrossRef] [Green Version]
- Stephens, O.W.; Zhang, Q.; Qu, P.; Zhou, Y.; Chavan, S.; Tian, E.; Williams, D.R.; Epstein, J.; Barlogie, B.; Shaughnessy, J.D., Jr. An intermediate-risk multiple myeloma subgroup is defined by sIL-6r: Levels synergistically increase with incidence of SNP rs2228145 and 1q21 amplification. Blood 2012, 119, 503–512. [Google Scholar] [CrossRef]
- Stone, K.; Woods, E.; Szmania, S.M.; Stephens, O.W.; Garg, T.K.; Barlogie, B.; Shaughnessy, J.D., Jr.; Hall, B.; Reddy, M.; Hoering, A.; et al. Interleukin-6 receptor polymorphism is prevalent in HIV-negative Castleman Disease and is associated with increased soluble interleukin-6 receptor levels. PLoS ONE 2013, 8, e54610. [Google Scholar] [CrossRef] [Green Version]
- Revez, J.A.; Bain, L.; Chapman, B.; Powell, J.E.; Jansen, R.; Duffy, D.L.; Tung, J.Y.; Penninx, B.W.; Visscher, P.M.; De Geus, E.J.; et al. A new regulatory variant in the interleukin-6 receptor gene associates with asthma risk. Genes Immun. 2013, 14, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, G.A.; Robinson, M.B.; Hastie, A.T.; Li, X.; Li, H.; Moore, W.C.; Howard, T.D.; Busse, W.W.; Erzurum, S.C.; Wenzel, S.E.; et al. The IL6R variation Asp(358)Ala is a potential modifier of lung function in subjects with asthma. J. Allergy Clin. Immunol. 2012, 130, 510–515.e1. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rodríguez, L.; Lamas, J.R.; Varadé, J.; López-Romero, P.; Tornero-Esteban, P.; Abasolo, L.; de la Concha, E.G.; Jover, J.A.; Urcelay, E.; Fernández-Gutiérrez, B. Plasma soluble IL-6 receptor concentration in rheumatoid arthritis: Associations with the rs8192284 IL6R polymorphism and with disease activity. Rheumatol. Int. 2001, 31, 409–413. [Google Scholar] [CrossRef]


| CTRL | DVT | p-Value | |
|---|---|---|---|
| Age (years), median (range) | 48 (41.7–62) | 56 (46–71) | 0.2392 * |
| Gender, number (%) | |||
| Male | 12 (54.5) | 12 (63.2) | 0.76 † |
| Female | 10 (45.5) | 7 (36.8) | |
| CRP, median (range) | 2.062 (0.79–6.96) | 6 (3–11) | 0.04 * |
| Parameter | DVT Patients Number (%) | Healthy Controls Number (%) | OR, CI p-Value |
|---|---|---|---|
| Plasma IL-6 | |||
| ≥3.465 | 9 (50) | 3 (15) | 5.7, 1.22–26.34 ≤0.05 |
| <3.465 | 9 (50) | 17 (85) | |
| IL6R rs2228145 genotype | |||
| AC and CC | 13 (68.42) | 14 (66.67) | 1.08, 0.29–4.08 1.00 |
| Wild type | 6 (31.58) | 7 (33.33) | |
| IL-6 and IL6R rs2228145 genotype | |||
| ≥3.465 and AC and CC | 6 (33.33) | 0 | 21.32, 1.10–412.20 ≤0.01 |
| ≥3.465 and WT; <3.465 and WT, AC, CC | 12 (66.67) | 20 (100) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salemi, R.; Gattuso, G.; Tomasello, B.; Lavoro, A.; Gaudio, A.; Libra, M.; Signorelli, S.S.; Candido, S. Co-Occurrence of Interleukin-6 Receptor Asp358Ala Variant and High Plasma Levels of IL-6: An Evidence of IL-6 Trans-Signaling Activation in Deep Vein Thrombosis (DVT) Patients. Biomolecules 2022, 12, 681. https://doi.org/10.3390/biom12050681
Salemi R, Gattuso G, Tomasello B, Lavoro A, Gaudio A, Libra M, Signorelli SS, Candido S. Co-Occurrence of Interleukin-6 Receptor Asp358Ala Variant and High Plasma Levels of IL-6: An Evidence of IL-6 Trans-Signaling Activation in Deep Vein Thrombosis (DVT) Patients. Biomolecules. 2022; 12(5):681. https://doi.org/10.3390/biom12050681
Chicago/Turabian StyleSalemi, Rossella, Giuseppe Gattuso, Barbara Tomasello, Alessandro Lavoro, Agostino Gaudio, Massimo Libra, Salvatore Santo Signorelli, and Saverio Candido. 2022. "Co-Occurrence of Interleukin-6 Receptor Asp358Ala Variant and High Plasma Levels of IL-6: An Evidence of IL-6 Trans-Signaling Activation in Deep Vein Thrombosis (DVT) Patients" Biomolecules 12, no. 5: 681. https://doi.org/10.3390/biom12050681