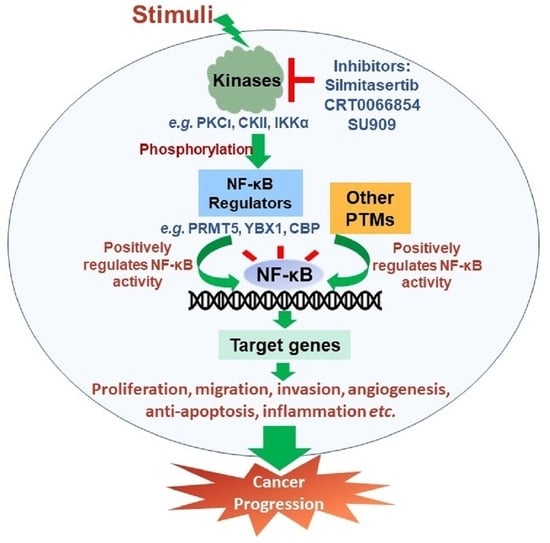
Phosphorylation of the Regulators, a Complex Facet of NF-κB Signaling in Cancer
Abstract
:1. Introduction
1.1. Brief Overview of Cancer and Key Signaling Pathways
1.2. Overview of NF-κB Signaling
1.3. Implication of NF-κB Signaling in Cancer
2. General Role of Phosphorylation of NF-κB Pathway in Cancer
3. Phosphorylation of Novel NF-κB Activators in Cancer
3.1. Phosphorylation of NF-κB Activator—PRMT5 in Cancer
3.1.1. PRMT5, a Novel Activator of NF-κB in Cancer
3.1.2. Effect of PRMT5 Phosphorylation on NF-κB Signaling in Cancer
3.2. Phosphorylation of NF-κB Activator—YBX1 in Cancer
3.2.1. YBX1, a Novel Activator of NF-κB
3.2.2. Effect of YBX1 Phosphorylation on NF-κB Signaling in Cancer
4. Phosphorylation of Other Known Regulators of NF-κB Pathway
5. Application of Phosphorylation of Regulators in NF-κB Signaling
6. Perspective and Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Sever, R.; Brugge, J.S. Signal Transduction in Cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a006098. [Google Scholar] [CrossRef] [Green Version]
- Freudlsperger, C.; Bian, Y.; Contag, S.; Burnett, J.; Coupar, J.; Yang, X.; Chen, Z.; Van Waes, C. TGF-β and NF-κB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene 2013, 32, 1549–1559. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuels, Y.; Ericson, K. Oncogenic PI3K and its role in cancer. Curr. Opin. Oncol. 2006, 18, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Baltimore, D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986, 46, 705–716. [Google Scholar] [CrossRef]
- Sen, R.; Baltimore, D. Inducibility of κ immunoglobulin enhancer-binding protein NF-κB by a posttranslational mechanism. Cell 1986, 47, 921–928. [Google Scholar] [CrossRef]
- Tripathi, P.; Aggarwal, A. NF-κB transcription factor: A key player in the generation of immune response. Curr. Sci. 2006, 90, 519. [Google Scholar]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. Leading Edge Review 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, C.; Bucci, I.; Napolitano, G. The role of the transcription factor Nuclear Factor-kappa B in thyroid autoimmunity and cancer. Front. Endocrinol. 2018, 9, 471. [Google Scholar] [CrossRef] [Green Version]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Stark, G.R. Cytokine overexpression and constitutive NF-κB in cancer. Cell Cycle 2004, 3, 1114–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.; Prabhu, L.; Hartley, A.-V.; Martin, M.; Sun, E.; Jiang, G.; Liu, Y.; Lu, T. Methylation of NF-κB and its Role in Gene Regulation. In Gene Expression and Regulation in Mammalian Cells—Transcription from General Aspects; IntechOpen: London, UK, 2018; p. 291. [Google Scholar]
- Sun, S.C. The noncanonical NF-κB pathway. Immunol. Rev. 2012, 246, 125–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-κB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Li, C.C.H. Association of Cdk2/cyclin E and NF-κB complexes at G1/S phase. Biochem. Biophys. Res. Commun. 1998, 249, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Krappmann, D.; Eichten, A.; Heder, A.; Scheidereit, C.; Strauss, M. NF-κB Function in Growth Control: Regulation of Cyclin D1 Expression and G0/G1-to-S-Phase Transition. Mol. Cell. Biol. 1999, 19, 2690–2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.; Hong, J. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef]
- Li, J.; Lau, G.K.-K.; Chen, L.; Dong, S.; Lan, H.-Y.; Huang, X.-R.; Li, Y.; Luk, J.M.; Yuan, Y.-F.; Guan, X. Interleukin 17A Promotes Hepatocellular Carcinoma Metastasis via NF-κB Induced Matrix Metalloproteinases 2 and 9 Expression. PLoS ONE 2011, 6, e21816. [Google Scholar]
- Huang, S.; Pettaway, C.A.; Uehara, H.; Bucana, C.D.; Fidler, I.J. Blockade of NF-κB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene 2001, 20, 4188–4197. [Google Scholar] [CrossRef] [Green Version]
- Inoue, J.I.; Gohda, J.; Akiyama, T.; Semba, K. NF-κB activation in development and progression of cancer. Cancer Sci. 2007, 98, 268–274. [Google Scholar] [CrossRef]
- Kim, Y.A.; Lee, W.H.; Choi, T.H.; Rhee, S.H.; Park, K.Y.; Choi, Y.H. Involvement of p21WAF1/CIP1, pRB, Bax and NF-kappaB in induction of growth arrest and apoptosis by resveratrol in human lung carcinoma A549 cells. Int. J. Oncol. 2003, 23, 1143–1149. [Google Scholar] [PubMed]
- Wang, C.Y.; Cusack, J.C.; Liu, R.; Baldwin, A.S. Control of inducible chemoresistance: Enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-κB. Nat. Med. 1999, 5, 412–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.-L.; Kamata, H.; Karin, M. IKK/NF-k κB signaling: Balancing life and death-a new approach to cancer therapy. J. Clin. Investig. 2005, 115, 2625–2632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevilla, L.; Zaldumbide, A.; Pognonec, P.; Boulukos, K.E. Transcriptional regulation of the bcl-x gene encoding the anti-apoptotic Bcl-xL protein by Ets, Rel/NFkappaB, STAT and AP1 transcription factor families. Histol. Histopathol. 2001, 16, 595–601. [Google Scholar] [PubMed]
- Tergaonkar, V.; Pando, M.; Vafa, O.; Wahl, G.; Verma, I. p53 stabilization is decreased upon NFκB activation: A role for NFκB in acquisition of resistance to chemotherapy. Cancer Cell 2002, 1, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, A.; Xu, M.; Chen, Z.J. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene 2007, 26, 3214–3226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Fujita, N.; Tsuruo, T. 3-Phosphoinositide-dependent protein kinase-1-mediated IκB kinase β (IKKB) phosphorylation activates NF-κB signaling. J. Biol. Chem. 2005, 280, 40965–40973. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Hagler, J.; Palombella, V.J.; Melandri, F.; Seherer, D.; Ballard, D.; Maniatis, T. Signal-induced site-specific phosphorylation targets IΚB x to the ubiquitin-proteasome pathway. Genes Dev. 1995, 9, 1586–1597. [Google Scholar] [CrossRef] [Green Version]
- Karin, M. The IκB kinase (IKK) complex as a critical regulator of immune responses. Int. Congr. Ser. 2005, 1285, 97–103. [Google Scholar] [CrossRef]
- Lis, C.-C.H.; Dais, R.-M.; Chens, E.; Longoll, D.L. Phosphorylation of NF-ΚBl-p50 Is Involved in NF-kappa B activation and stable DNA Binding. J. Biochem. 1994, 269, 30089–30092. [Google Scholar]
- Martin, A.G.; Fresno, M. Tumor necrosis factor-α activation of NF-κB requires the phosphorylation of Ser-471 in the transactivation domain of c-Rel. J. Biol. Chem. 2000, 275, 24383–24391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.; Hartley, A.-V.; Jin, J.; Sun, M.; Lu, T. Phosphorylation of NF-κB in Cancer. InTechOpen, 2019. Available online: www.intechopen.com (accessed on 20 October 2020).
- Ling, L.; Cao, Z.; Goeddel, D.V. Nf-κB-inducing kinase activates IKK-α by phosphorylation of Ser-176. Proc. Natl. Acad. Sci. USA 1998, 95, 3792–3797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, G.; Fong, A.; Sun, S.C. Induction of p100 processing by NF-κB-inducing kinase involves docking IκB kinase α (IKKα) to p100 and IKKα-mediated phosphorylation. J. Biol. Chem. 2004, 279, 30099–30105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, F.; Smith, E.; Carmody, R. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Bonizzi, G.; Seagroves, T.N.; Greten, F.R.; Johnson, R.; Schmidt, E.V.; Karin, M. IKKα provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 2001, 107, 763–775. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wei, H.; Prabhu, L.; Zhao, W.; Martin, M.; Hartley, A.V.; Lu, T. Role of Novel Serine 316 Phosphorylation of the p65 Subunit of NF-κB in Differential Gene Regulation. J. Boil. Chem. 2015, 290, 20336–20347. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Shao, L.; Creighton, C.J.; Zhang, Y.; Xin, L.; Ittmann, M.; Wang, J. Function of phosphorylation of NF-κB p65 ser536 in prostate cancer oncogenesis. Oncotarget 2015, 6, 6281. [Google Scholar] [CrossRef] [Green Version]
- Ho, S.H.; Ali, A.; Chin, T.M.; Go, M.L. Dioxonaphthoimidazoliums AB1 and YM155 disrupt phosphorylation of p50 in the NF-κB pathway. Oncotarget 2016, 7, 11625–11636. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Mundade, R.; Lange, K.C.; Lu, T. Protein arginine methylation of non-histone proteins and its role in diseases. Cell Cycle 2014, 13, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Bedford, M.T. Arginine methylation at a glance. J. Cell Sci. 2007, 120 Pt 24, 4243–4246. [Google Scholar] [CrossRef] [Green Version]
- Stopa, N.; Krebs, J.E.; Shechter, D. The PRMT5 arginine methyltransferase: Many roles in development, cancer and beyond. Cell. Mol. Life Sci. 2015, 72, 2041–2059. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Q.; Liu, C.; Han, F.; Chen, M.; Zhang, L.; Cui, X.; Qin, Y.; Bao, S.; Gao, F. Protein arginine methyltransferase 5 (Prmt5) is required for germ cell survival during mouse embryonic development. Biol. Reprod. 2015, 92, 104. [Google Scholar] [CrossRef] [PubMed]
- Tee, W.W.; Pardo, M.; Theunissen, T.W.; Yu, L.; Choudhary, J.S.; Hajkova, P.; Surani, M.A. Prmt5 is essential for early mouse development and acts in the cytoplasm to maintain ES cell pluripotency. Genes Dev. 2010, 24, 2772–2777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Yi, B.; Sun, J. Inhibition of cardiomyocyte hypertrophy by protein arginine methyltransferase 5. J. Biol. Chem. 2014, 289, 24325–24335. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Ronai, Z.A. PRMT5 function and targeting in cancer. Cell Stress 2020, 4, 199–215. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Wang, B.; Miyagi, M.; She, Y.; Gopalan, B.; Huang, D.B.; Ghosh, G.; Stark, G.R.; Lu, T. PRMT5 dimethylates R30 of the p65 subunit to activate NF-kappaB. Proc. Natl. Acad. Sci. USA 2013, 110, 13516–13521. [Google Scholar] [CrossRef] [Green Version]
- Hartley, A.-V.; Wang, B.; Jiang, G.; Wei, H.; Sun, M.; Prabhu, L.; Martin, M.; Safa, A.; Sun, S.; Liu, Y.; et al. Regulation of a PRMT5/NF-κB Axis by Phosphorylation of PRMT5 at Serine 15 in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 3684. [Google Scholar] [CrossRef]
- Prabhu, L.; Wei, H.; Chen, L.; Demir, Ö.; Sandusky, G.; Sun, E.; Wang, J.; Mo, J.; Zeng, L.; Fishel, M.; et al. Adapting AlphaLISA high throughput screen to discover a novel small-molecule inhibitor targeting protein arginine methyltransferase 5 in pancreatic and colorectal cancers. Oncotarget 2017, 8, 39963–39977. [Google Scholar] [CrossRef] [Green Version]
- Eliseeva, I.A.; Kim, E.R.; Guryanov, S.G.; Ovchinnikov, L.P.; Lyabin, D.N. Y-box-binding protein 1 (YB-1) and its functions. Biochemistry 2011, 76, 1402–1433. [Google Scholar] [CrossRef]
- Uchiumi, T.; Fotovati, A.; Sasaguri, T.; Shibahara, K.; Shimada, T.; Fukuda, T.; Nakamura, T.; Izumi, H.; Tsuzuki, T.; Kuwano, M.; et al. YB-1 Is important for an early stage embryonic development neural tube formation and cell proliferation. J. Biol. Chem. 2006, 281, 40440–40449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohno, K.; Izumi, H.; Uchiumi, T.; Ashizuka, M.; Kuwano, M. The pleiotropic functions of the Y-box-binding protein, YB-1. BioEssays 2003, 25, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, M.; Oda, Y.; Izumi, H.; Yang, S.J.; Uchiumi, T.; Iwamoto, Y.; Toi, M.; Fujii, T.; Yamana, H.; Kinoshita, H.; et al. The role of nuclear Y-box binding protein 1 as a global marker in drug resistance. Mol. Cancer Ther. 2004, 3, 1485–1492. [Google Scholar] [PubMed]
- Prabhu, L.; Mundade, R.; Wang, B.; Wei, H.; Hartley, A.-V.; Martin, M.; McElyea, K.; Temm, C.J.; Sandusky, G.; Liu, Y.; et al. Critical role of phosphorylation of serine 165 of YBX1 on the activation of NF-κB in colon cancer. Oncotarget 2015, 6, 29396–29412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.; Hua, L.; Wang, B.; Wei, H.; Prabhu, L.; Hartley, A.-V.; Jiang, G.; Liu, Y.; Lu, T. Novel Serine 176 Phosphorylation of YBX1 Activates NF-κB in Colon Cancer. J. Biol. Chem. 2017, 292, 3433–3444. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Su, J.; Wang, Y.; Fu, D.; Ideozu, J.E.; Geng, H.; Cui, Q.; Wang, C.; Chen, R.; Yu, Y.; et al. The interaction of YBX1 with G3BP1 promotes renal cell carcinoma cell metastasis via YBX1/G3BP1-SPP1- NF-κB signaling axis. J. Exp. Clin. Cancer Res. 2019, 38, 386. [Google Scholar] [CrossRef] [Green Version]
- Blenkiron, C.; Hurley, D.G.; Fitzgerald, S.; Lasham, A. Links between the oncoprotein YB-1 and small non-coding RNAs in breast cancer. PLoS ONE 2013, 8, e80171. [Google Scholar] [CrossRef]
- Shibata, T.; Watari, K.; Kawahara, A.; Sudo, T.; Hattori, S.; Murakami, Y.; Izumi, H.; Itou, J.; Toi, M.; Akiba, J.; et al. Targeting Phosphorylation of Y-Box—Binding Protein YBX1 by TAS0612 and Everolimus in Overcoming Antiestrogen Resistance. Mol. Cancer Ther. 2020, 19, 882–894. [Google Scholar] [CrossRef]
- Sutherland, B.W.; Kucab, J.; Wu, J.; Lee, C.; Cheang, M.C.U.; Yorida, E.; Turbin, D.; Dedhar, S.; Nelson, C.; Pollak, M.; et al. Akt phosphorylates the Y-box binding protein 1 at Ser102 located in the cold shock domain and affects the anchorage-independent growth of breast cancer cells. Oncogene 2005, 24, 4281–4292. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Ding, D.; Yan, Y.; Li, H.; Wang, B.; Ma, L.; Ye, Z.; Ma, T.; Wu, Q.; Rodrigues, D.N.; et al. Phosphorylated RB Promotes Cancer Immunity by Inhibiting NF-κB Activation and PD-L1 Expression. Mol. Cell 2019, 73, 22–35.e6. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Lu, P.D.; Zhang, Y.; Scheuner, D.; Kaufman, R.J.; Sonenberg, N.; Harding, H.P.; Ron, D. Translational Repression Mediates Activation of Nuclear Factor Kappa B by Phosphorylated Translation Initiation Factor 2. Mol. Cell. Biol. 2004, 24, 10161–10168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arun, P.; Brown, M.; Ehsanian, R.; Chen, Z.; Van Waes, C. Nuclear NF-κB p65 phosphorylation at Serine 276 by protein kinase A contributes to the malignant phenotype of head and neck cancer. Clin. Cancer Res. 2009, 15, 5974–5984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCOY, C.E.; Campbell, D.G.; Deak, M.; Bloomberg, G.B.; Arthur, J.S.C. MSK1 activity is controlled by multiple phosphorylation sites. Biochem. J. 2005, 387 Pt 2, 507–517. [Google Scholar] [CrossRef]
- Vermeulen, L.; De Wilde, G.; Van Damme, P.; Vanden Berghe, W.; Haegeman, G. Transcriptional activation of the NF-κB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J. 2003, 22, 1313–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.-C.; Ju, T.-K.; Hung, M.-C.; Chen, C.-C. Phosphorylation of CBP by IKKα Promotes Cell Growth by Switching the Binding Preference of CBP from p53 to NF-κB. Mol. Cell 2007, 26, 75–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reipas, K.M.; Law, J.H.; Couto, N.; Islam, S.; Li, Y.; Li, H.; Cherkasov, A.; Jung, K.; Cheema, A.S.; Jones, S.J.M.; et al. Luteolin is a novel p90 ribosomal S6 kinase (RSK) inhibitor that suppresses Notch4 signaling by blocking the activation of Y-box binding protein-1 (YB-1). Oncotarget 2013, 4, 329–345. [Google Scholar] [CrossRef]
- Hoberg, J.E.; Yeung, F.; Mayo, M.W. SMRT Derepression by the IκB Kinase α: A Prerequisite to NF-κB Transcription and Survival. Mol. Cell 2004, 16, 245–255. [Google Scholar] [CrossRef]
- Shembade, N.; Pujari, R.; Harhaj, N.S.; Abbott, D.W.; Harhaj, E.W. The kinase IKKα inhibits activation of the transcription factor NF-κB by phosphorylating the regulatory molecule TAX1BP1. Nat. Immunol. 2011, 12, 834–843. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Wei, L.; Wu, B. PRMT5 Promotes Aerobic Glycolysis and Invasion of Breast Cancer Cells by Regulating the LXRα/NF-κBp65 Pathway. OncoTargets Ther. 2020, 13, 3347–3357. [Google Scholar] [CrossRef] [Green Version]
- Hartley, A.-V.; Wang, B.; Mundade, R.; Jiang, G.; Sun, M.; Wei, H.; Sun, S.; Liu, Y.; Lu, T. PRMT5-mediated methylation of YBX1 regulates NF-κB activity in colorectal cancer. Sci. Rep. 2020, 10, 15934. [Google Scholar] [CrossRef]
- Raafat, A.; Bargo, S.; McCurdy, D.; Callahan, R. The ANK repeats of Notch-4/Int3 activate NF-κB canonical pathway in the absence of Rbpj and causes mammary tumorigenesis. Sci. Rep. 2017, 7, 13690. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Cho, Y.-Y.; Zhu, F.; Xu, Y.-M.; Wen, W.; Ma, W.-Y.; Bode, A.M.; Dong, Z. RSK2 mediates NF-κB activity through the phosphorylation of IκBα in the TNF-R1 pathway. FASEB J. 2010, 24, 3490–3499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buss, H.; Handschick, K.; Jurrmann, N.; Pekkonen, P.; Beuerlein, K.; Müller, H.; Wait, R.; Saklatvala, J.; Ojala, P.M.; Schmitz, M.L.; et al. Cyclin-Dependent Kinase 6 Phosphorylates NF-κB P65 at Serine 536 and Contributes to the Regulation of Inflammatory Gene Expression. PLoS ONE 2012, 7, e51847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthony, N.G.; Baiget, J.; Berretta, G.; Boyd, M.; Breen, D.; Edwards, J.; Gamble, C.; Gray, A.I.; Harvey, A.L.; Hatziieremia, S.; et al. Inhibitory Kappa B Kinase α (IKKα) Inhibitors That Recapitulate Their Selectivity in Cells against Isoform-Related Biomarkers. J. Med. Chem. 2017, 60, 7043–7066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manni, S.; Brancalion, A.; Mandato, E.; Tubi, L.Q.; Colpo, A.; Pizzi, M.; Cappellesso, R.; Zaffino, F.; Maggio, S.A.D.; Cabrelle, A.; et al. Protein Kinase CK2 Inhibition Down Modulates the NF-κB and STAT3 Survival Pathways, Enhances the Cellular Proteotoxic Stress and Synergistically Boosts the Cytotoxic Effect of Bortezomib on Multiple Myeloma and Mantle Cell Lymphoma Cells. PLoS ONE 2013, 8, e75280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergote, I.; Heitz, F.; Buderath, P.; Powell, M.; Sehouli, J.; Lee, C.M.; Hamilton, A.; Fiorica, J.; Moore, K.N.; Teneriello, M.; et al. A randomized, double-blind, placebo-controlled phase 1b/2 study of ralimetinib, a p38 MAPK inhibitor, plus gemcitabine and carboplatin versus gemcitabine and carboplatin for women with recurrent platinum-sensitive ovarian cancer. Gynecol. Oncol. 2020, 156, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Plevin, R.; Al-Obaidi, A.; Khalaf, Y.; Mackay, S.; Paul, A. Novel selective inhibitors of inhibitory kappa B kinase alpha-new drugs for the treatment of Cancer. In Proceedings for Annual Meeting of the Japanese Pharmacological Society WCP2018 (The 18th World Congress of Basic and Clinical Pharmacology), Kyoto, Japan, 1–6 July 2018; Japanese Pharmacological Society: Kyoto, Japan, 2018; p. OR7-1. [Google Scholar]
- Baud, V.; Karin, M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef] [Green Version]


| NF-κB Regulators | Phosphorylation Site | Kinase | Function on NF-κB Activity | Reference |
|---|---|---|---|---|
| PRMT5 | Ser15 | PKCι | Enhances NF-κB | [50] |
| YBX1 | Ser 102 Ser165 and Ser176 | RSK CKI and CKII | Enhances NF-κB | [56,57,68] |
| CBP | Ser1382 and Ser1386 | IKKα | Enhances NF-κB | [67] |
| SMRT | Ser2410 | IKKα | Enhances NF-κB | [69] |
| MSK1 | Thr581 and Ser360 | P38 | Enhances NF-κB | [66] |
| TAX1BP1 | Ser593 and Ser624 | IKKα | Suppresses NF-κB | [70] |
| eIF2α | Ser51 | PKR, PERK, GCN2, or HRI | Enhances NF-κB | [63] |
| Rb | serine-249 and threonine-252 | CDK4/6 | Suppresses NF-κB | [62] |
| Kinase Inhibitor | Kinase | NF-κB Regulators | Stage in Drug Development | Type of Cancer | Reference |
|---|---|---|---|---|---|
| CX-4945 (Silmitasertib) K27 | CKII | YBX1 | Phase I/II clinical trial Preclinical | Medulloblastoma Mantle cell lymphoma and Multiple myeloma | ClinicalTrials.gov Identifier: [NCT03904862] [77] |
| LY2228820 (Ralimetinib) | P38 | MSK1 | Phase 1b/2 clinical trial in combination with gemcitabine and carboplatin | Ovarian cancer | ClinicalTrials.gov Identifier: [NCT01663857] [78] |
| CRT0066854 | PKCι | PRMT5 | Preclinical | Colon cancer cell lines | [50] |
| Compound 48 (proprietary code SU909) | IKKα | p100, TAX1BP1, SMRT, CBP | Preclinical | Osteosarcoma and pancreatic cancer cell lines | [76,79] |
| Luteolin | RSK | YBX1 | Preclinical | Triple negative breast cancer cell lines | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motolani, A.; Martin, M.; Sun, M.; Lu, T. Phosphorylation of the Regulators, a Complex Facet of NF-κB Signaling in Cancer. Biomolecules 2021, 11, 15. https://doi.org/10.3390/biom11010015
Motolani A, Martin M, Sun M, Lu T. Phosphorylation of the Regulators, a Complex Facet of NF-κB Signaling in Cancer. Biomolecules. 2021; 11(1):15. https://doi.org/10.3390/biom11010015
Chicago/Turabian StyleMotolani, Aishat, Matthew Martin, Mengyao Sun, and Tao Lu. 2021. "Phosphorylation of the Regulators, a Complex Facet of NF-κB Signaling in Cancer" Biomolecules 11, no. 1: 15. https://doi.org/10.3390/biom11010015