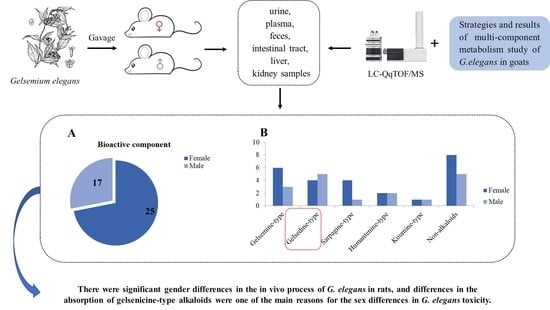
Sex Differences in the In Vivo Exposure Process of Multiple Components of Gelsemium elegans in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Herb Material and Reagents
2.2. Animals and Administration
2.3. Sample Preparation
2.4. Analysis Conditions for Metabolic Studies
3. Results
3.1. Comparative Analysis of Urine Samples from Male and Female Rats
3.2. Comparative Analysis of Plasma Samples from Male and Female Rats
3.3. Comparative Analysis of Fecal Samples from Male and Female Rats
3.4. Comparative Analysis of Tissue Samples from Male and Female Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, G.L.; Su, Y.P.; Liu, M.; Xu, Y.; Yang, J.; Liao, K.J.; Yu, C.X. Medicinal plants of the genus Gelsemium (Gelsemiaceae, Gentianales)—A review of their phytochemistry, pharmacology, toxicology and traditional use. J. Ethnopharmacol. 2014, 152, 33–52. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.Y.; Wang, G.Y.; Li, N.P.; Chen, M.F.; Gu, J.H.; Zhang, D.M.; Wang, L.; Ye, W.C. Five new koumine-type alkaloids from the roots of Gelsemium elegans. Fitoterapia 2017, 118, 112–117. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lin, L.; Cheng, P.; Sun, Z.L.; Wu, Y.; Liu, Z.Y. Fingerprint analysis of Gelsemium elegans by HPLC followed by the targeted identification of chemical constituents using HPLC coupled with quadrupole-time-of-flight mass spectrometry. Fitoterapia 2017, 121, 94–105. [Google Scholar] [CrossRef]
- King, S.R. International Collation of Traditional and Folk Medicine: Northeast Asia. Part 1 by Takeatsu Kimura; World Scientific Publishing Co., Inc.: River Edge, NJ, USA, 1996; p. 221. ISBN 981022589X. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, H.Q.; Liu, H.; Liu, M.; Huang, Z.Y.; Yang, J.; Su, Y.P.; Yu, C.X. Effects of koumine, an alkaloid of Gelsemium elegans Benth., on inflammatory and neuropathic pain models and possible mechanism with allopregnanolone. Pharmacol. Biochem. Behav. 2012, 101, 504–514. [Google Scholar] [CrossRef]
- Bellavite, P.; Magnani, P.; Zanolin, E.; Conforti, A. Homeopathic Doses of Gelsemium sempervirens Improve the Behavior of Mice in Response to Novel Environments. Evid.-Based Complement. Altern. Med. 2011, 2011, 362517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, Q.; Liu, M.; Wu, M.X.; Xu, Y.; Yang, J.; Huang, H.H.; Yu, C.X. Anti-allodynic and Neuroprotective Effects of Koumine, a Benth Alkaloid, in a Rat Model of Diabetic Neuropathy. Biol. Pharm. Bull. 2014, 37, 858–864. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Huang, H.-H.; Yang, J.; Su, Y.-P.; Lin, H.-W.; Lin, L.-Q.; Liao, W.-J.; Yu, C.-X. The active alkaloids of Gelsemium elegans Benth. are potent anxiolytics. Psychopharmacology 2013, 225, 839–851. [Google Scholar] [CrossRef]
- Meyer, L.; Boujedaini, N.; Pattemensah, C.; Mensahnyagan, A.G. Pharmacological effect of gelsemine on anxiety-like behavior in rat. Behav. Brain Res. 2013, 253, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Gao, B.; Luo, D.; Wen, Y.; Ma, X. Apoptotic Effect of Koumine on Human Breast Cancer Cells and the Mechanism Involved. Cell Biochem. Biophys. 2015, 72, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, R.K.; Miglani, A.; Gupta, M.; Meena, B.S.; Chadha, V.; Joseph, F.; Kalsi, A.; Konthembath, P.; Sharma, K.; Rama, K.N.; et al. Homeopathic Remedies in COVID-19: Prognostic Factor Research. Homeopathy 2021, 110, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Masiello, D.J. The COVID-19 Pandemic: A View from New York City. Homeopathy 2020, 109, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.G. The Experience of an Italian Public Homeopathy Clinic during the COVID-19 Epidemic, March-May 2020. Homeopathy 2020, 109, 167–168. [Google Scholar] [CrossRef]
- Karwacki, Z.; Niewiadomski, S.; Rzaska, M.; Witkowska, M. The effect of bispectral index monitoring on anaesthetic requirements in target-controlled infusion for lumbar microdiscectomy. Anaesthesiol. Intensive Ther. 2014, 46, 284–288. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, L.; Zhong, Y.; Fang, X.; Liu, Y.; Chen, H.; Zhang, W. Gelsemium elegans Poisoning: A Case with 8 Months of Follow-up and Review of the Literature. Front. Neurol. 2017, 8, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, D.; Qiao, D.F.; Chen, X.C.; Feng, C.Q.; Luo, Q.Z.; Tan, X.H. Fatal poisoning by accidental ingestion of the "heartbreak grass" (Gelsemium elegans) verified by toxicological and medico-legal analyses. Forensic. Sci. Int. 2021, 321, 110745. [Google Scholar] [CrossRef]
- Liu, M.; Shen, J.; Liu, H.; Xu, Y.; Su, Y.P.; Yang, J.; Yu, C.X. Gelsenicine from Gelsemium elegans attenuates neuropathic and inflammatory pain in mice. Biol. Pharm. Bull. 2011, 34, 1877–1880. [Google Scholar] [CrossRef] [Green Version]
- Rujjanawate, C.; Kanjanapothi, D.; Panthong, A. Pharmacological effect and toxicity of alkaloids from Gelsemium elegans Benth. J. Ethnopharmacol. 2003, 89, 91–95. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Sun, F.; Zhang, J.; Jin, Y.; Li, Y.; Zhou, J.; Li, Y.; Zhu, K. Gelsedine-type alkaloids: Discovery of natural neurotoxins presented in toxic honey. J. Hazard. Mater. 2020, 381, 120999. [Google Scholar] [CrossRef]
- Zuo, M.-T.; Wang, Z.-Y.; Yang, K.; Li, Y.-J.; Huang, C.-Y.; Liu, Y.-C.; Yu, H.; Zhao, X.-J.; Liu, Z.-Y. Characterization of absorbed and produced constituents in goat plasma urine and faeces from the herbal medicine Gelsemium elegans by using high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Ethnopharmacol. 2020, 252, 112617. [Google Scholar] [CrossRef]
- Liu, Y.C.; Xiao, S.; Yang, K.; Ling, L.; Sun, Z.L.; Liu, Z.Y. Comprehensive identification and structural characterization of target components from Gelsemium elegans by high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry based on accurate mass databases combined with MS/MS spectra. J. Mass Spectrom. 2017, 52, 378–396. [Google Scholar] [CrossRef]
- Xiang, Z.; Qiu, J.; He, X.; Yu, X. Toxicokinetics, in vivo metabolic profiling, and in vitro metabolism of gelsenicine in rats. Arch. Toxicol. 2022, 96, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.-J.; Zuo, M.-T.; Huang, S.-J.; Ma, X.; Wang, Z.-Y.; Liu, Z.-Y. Metabolic profile and tissue distribution of Humantenirine, an oxindole alkaloid from Gelsemium, after oral administration in rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1181, 122901. [Google Scholar] [CrossRef]
- Cao, J.-J.; Yang, K.; Yu, H.; Long, X.-M.; Li, Y.-J.; Sun, Z.-L.; Liu, Z.-Y. Comparative toxicokinetic profiles of multiple-components of Gelsemium elegans in pigs and rats after a single oral administration. Toxicon 2020, 181, 28–35. [Google Scholar] [CrossRef]
- Ye, L.-X.; Xu, Y.; Zhang, S.-H.; Cao, D.-X.; Chen, L.-F.; Su, Y.-P.; Huang, H.-H.; Yu, C.-X. Orally Administered Koumine Persists Longer in the Plasma of Aged Rats Than That of Adult Rats as Assessed by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Front. Pharmacol. 2020, 11, 1113. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Z.-Y.; Zuo, M.-T.; Yang, K.; Sun, Z.-L.; Wu, Y.; Liu, Z.-Y. Excretion, Metabolism, and Tissue Distribution of Gelsemium elegans ( Gardn. & Champ.) Benth in Pigs. Molecules 2022, 27, 2605. [Google Scholar] [CrossRef]
- Huang, S.-J.; Zuo, M.-T.; Qi, X.-J.; Huang, C.-Y.; Liu, Z.-Y. Phosphoproteomics reveals NMDA receptor-mediated excitotoxicity as a key signaling pathway in the toxicity of gelsenicine. Food Chem. Toxicol. 2021, 156, 112507. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Yang, K.; Long, X.-M.; Xiao, G.; Huang, S.-J.; Zeng, Z.-Y.; Liu, Z.-Y.; Sun, Z.-L. Toxicity assessment of gelsenicine and the search for effective antidotes. Hum. Exp. Toxicol. 2022, 41, 9603271211062857. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.-T.; Wu, Y.; Wang, Z.-Y.; Wang, N.; Huang, S.-J.; Yu, H.; Zhao, X.-J.; Huang, C.-Y.; Liu, Z.-Y. A comprehensive toxicity evaluation in rats after long-term oral Gelsemium elegans exposure. Biomed. Pharmacother. 2021, 137, 111284. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Sun, R.; Chen, M.; Hu, Y.; Lan, Y.; Gan, L.; You, G.; Yue, M.; Wang, H.; Xia, B.; Zhao, J.; et al. CYP3A4/5 mediates the metabolic detoxification of humantenmine, a highly toxic alkaloid from Gelsemium elegans Benth. J. Appl. Toxicol. 2019, 39, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Zuo, M.-T.; Zhao, X.-J.; Li, Y.-J.; Sun, Z.-L.; Liu, Z.-Y. Comparative metabolism of gelsenicine in liver microsomes from humans, pigs, goats and rats. Rapid Commun. Mass Spectrom. RCM 2020, 34, e8843. [Google Scholar] [CrossRef]
- Toutain, P.-L.; Ferran, A.; Bousquet-Mélou, A. Species differences in pharmacokinetics and pharmacodynamics. Handb. Exp. Pharmacol. 2010, 19–48. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. Implications of hepatic cytochrome P450-related biotransformation processes in veterinary sciences. Eur. J. Pharmacol. 2008, 585, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Urano, A.; Kogure, N.; Takayama, H.; Aimi, N. New oxindole alkaloids and iridoid from Carolina jasmine (Gelsemium sempervirens Ait. f.). Chem. Pharm. Bull. 2003, 51, 1211–1214. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Guo, T.; Wang, M.; Zhao, Q. Comparative study on the anti-neoplastic effect of non- alkaloid components from Gelsemium elegans Benth. in vitro and in vivo. China Pharm. 2006, 23, 1776–1778. [Google Scholar]
- Bhattacharyya, S.S.; Paul, S.; Mandal, S.K.; Banerjee, A.; Boujedaini, N.; Khuda-Bukhsh, A.R. A synthetic coumarin (4-methyl-7 hydroxy coumarin) has anti-cancer potentials against DMBA-induced skin cancer in mice. Eur. J. Pharmacol. 2009, 614, 128–136. [Google Scholar] [CrossRef] [PubMed]



| No. | Compound Class | Structures | Name | Groups/Proposed Metabolism | RT | Molecular Formula | [M + H]+ (m/z) | Error (ppm) | Rats (Female) | Rats (Male) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Alkaloid, Gelsemine-type |  | Unknown(H1) | R1 = R2 = R4 = R5 = R6 = H, R3 = CH2 | 4.668 | C19H20N2O2 | 309.1613 | −5.02 | U (5.0 × 104) P (1.5 × 103) F (1.2 × 104) | |
| 2 | Gelsemine(H2) | R1 = R2 = R4 = R6 = H, R3 = R5 = CH2 | 4.867 | C20H22N2O2 | 323.1782 | −8.68 | U (5.2 × 105) P (4.0 × 103) F (3.2 × 104) L (4.0 × 104) K (2.0 × 104) | U (1.0 × 104) P (8.0 × 102) K (1.5 × 104) | ||
| 3 | H2-M1 | +GlcA | 1.625 | C26H30N2O8 | 499.2037 | 7.61 | I (1.3 × 104) | |||
| 5 | H2-M2 | +O | 4.570 | C20H22N2O3 | 339.1685 | 5.38 | F (9.0 × 103) | U (1.2 × 104) | ||
| 6 | 19R-Hydroxydihydrogelsemine(H3) | R1 = R2 = R6 = H, R3 = OH, R4 = R5 = CH3 | 5.269 | C20H24N2O3 | 341.1868 | −2.44 | U (2.0 × 104) F (8.0 × 104) | U (1.0 × 104) F (3.5 × 104) | ||
| 8 | H3-M1 | +O | 4.668 | C20H24N2O4 | 357.1793 | 4.45 | U (8.0 × 103) | |||
| 9 | Gelsemine Oxide(H4) | R1 = R2 = R4 = R6 = H, R3 = CH3 oxide, R5 = CH3 | 5.178 | C20H22N2O3 | 339.1697 | 1.83 | U (1.2 × 104) | |||
| 10 | 19S-Hydroxydihydrogelsemine(H5) | R1 = R2 = R6 = H, R3 = OH, R4 = R5 = CH3 | 8.184 | C20H24N2O3 | 341.1880 | −5.97 | U (3.0 × 105) F (4.0 × 105) | U (1.4 × 105) F (1.6 × 105) | ||
| 11 | 21-Oxogelsemine(H6) | R1 = R2 = R4 = H, R4 = CH2, R5 = CH3, R6 = O | 12.707 | C20H20N2O3 | 337.1562 | −4.55 | P (3.0 × 103) | |||
| 12 | H6-M1 | +2H | 5.472 | C20H22N2O3 | 339.1732 | −8.52 | U (7.5 × 103) P (1.5 × 103) | |||
| 13 | H6-M2 | +O | 9.088 | C20H20N2O4 | 353.1515 | −5.44 | U (7.0 × 103) | |||
| 14 | Alkaloid, Gelsedine-type |  | 11-Hydroxygelsenicine(H7) | R1 = OH, R2 = R5 = H, R3 = N, R4 = C(CH2CH3) | 4.869 | C19H22N2O4 | 343.1680 | −8.08 | U (1.0 × 105) | U (1.0 × 104) |
| 15 | 14-Hydroxygelsenicine(H8) | R1 = R5 = H, R2 = OH, R3 = N, R4 = C(CH2CH3) | 5.269 | C19H22N2O4 | 343.1652 | 0.10 | U (1.2 × 105) P (1.0 × 103) L (1.3 × 104) | P (3.0 × 103) L (3.8 × 104) | ||
| 16 | Gelsenicine(H9) | R1 = R2 = R4 = H, R3 = N, R5 = CH3 | 8.990 | C19H22N2O3 | 327.1693 | 2.82 | F (2.1 × 105) L (4.0 × 104) | F (6.0 × 104) L (8.0 × 103) K (1.2 × 104) | ||
| 17 | Hydroxyl of gelsenicine(H10) | unknown | 10.598 | C19H22N2O4 | 343.1678 | −7.10 | I (1.3 × 104) | |||
| 18 | 19-Oxogelsencine(H11) | R1 = R2 = R5 = H, R3 = N, R4 = C(COCH3) | 19.743 | C19H20N2O4 | 341.1526 | −8.87 | U (2.5 × 104) | U (1.1 × 104) | ||
| 19 | Alkaloid, Sarpagine-type |  | Unkonw(H12) | unknown | 7.279 | C20H22N2O4 | 355.1684 | −8.94 | U (3.0 × 104) | U (1.3 × 104) |
| 20 | Dehydrokoumidine(H13) | R1 = R2 = R3 = R6 = H, R4 = O, R5 = N | 6.676 | C19H20N2O | 293.1638 | 3.56 | U (1.4 × 104) L (2.8 × 104) K (8.0 × 103) | |||
| 21 | Unknown(H14) | Unknown | 10.196 | C19H20N2O | 293.1652 | −1.23 | F (1.3 × 104) | |||
| 22 | Gardnerine(H15) | R1 = OCH3, R2 = R3 = R6 = H, R4 = CH2OH, R5 = N | 8.286 | C20H24N2O2 | 325.1926 | −4.77 | I (7.0 × 104) | |||
| 23 | Alkaloid, Humantenine-type |  | 14-Hydroxyrankinidine(H16) | R1 = R2 = R3 = R4 = H, R5 = CHCH3, R6 = OH | 10.898 | C20H24N2O4 | 357.1813 | −1.17 | U (4.0 × 104) F (4.0 × 104) L (7.0 × 103) | U (2.5 × 104) |
| 24 | Humantenine(H17) | R1 = R2 = R4 = R6 = H, R3 = CH3, R5 = CHCH3 | 13.613 | C21H26N2O3 | 355.2034 | −5.03 | F (1.5 × 104) L (2.5 × 104) K (1.0 × 104) | F (1.0 × 104) L (3.0 × 103) K (7.0 × 103) | ||
| 25 | Alkaloid, Koumine-type |  | Koumine(H18) | \ | 6.475 | C20H22N2O | 307.1817 | −3.95 | U (2.8 × 104) P (8.0 × 102) I (3.5 × 104)K (1.0 × 104) | U (1.0 × 104) P (7.0 × 102) I (6.0 × 104) L (6.0 × 103) K (1.3 × 104) |
| 26 | Iridoids |  | Geleganoid A/GRIR-1(H19) | R1 = R2 = OH, R3, R4 = OCH(OH) | 1.657 | C10H14O6 | 231.0878 | −6.46 | P (1.5 × 104) | |
| 27 | 7-Hydroxygelsemiol/9- Hydroxygelsemiol(H2O) | R1 = H, R2 = R3 = OH, R4 = CH2OH | 2.159 | C10H16O5 | 217.1050 | 9.49 | P (2.6 × 104) | |||
| 28 | 9-Hydroxysemperoside(H21) | R1 = H, R2 = OH, R3, R4 = OCH(OCH2CHOHC4H7O4) | 5.872 | C16H24O10 | 377.1468 | −6.85 | U (7.2 × 105) P (7.0 × 102) L (1.5 × 106) K (1.1 × 106) | U (5.0 × 105) P (7.0 × 102) F (3.0 × 104) L (1.3 × 106) K (1.5 × 106) | ||
| 29 | GSIR-1(H22) | R1 = R2 = H, R3 = OH, R4 = CH2 | 3.862 | C10H14O3 | 183.1027 | −6.20 | P (1.2 × 103) F (7.0 × 103) L (7.0 × 103) | P (1.5 × 103) F (6.0 × 103) | ||
| 30 | Unknow(H23) | Unknown | 3.058 | C10H12O3 | 171.1011 | 2.77 | U (1.2 × 104) | |||
| 31 | 7-Deoxygelsemide/9-Deoxygelsemide(H24) | R1/R2 = OH, R2/R1 = H, R3, R4 = OCH | 4.969 | C10H12O4 | 197.0825 | −8.49 | U (1.0 × 104) | |||
| 32 | 9-DeoxyGRIR-2(H25) | R1 = R2 = H, R3, R4 = OCH(OH) | 6.276 | C10H14O4 | 199.0947 | 9.01 | I (2.5 × 104) | |||
| 33 | Isomer of 7-Deoxygelsemide/9-Deoxygelsemide(H26) | R1/R2 = OH, R2/R1 = H, R3 = OH, R4 = CH2OH | 6.678 | C10H16O5 | 217.1078 | −3.47 | I (4.0 × 104) L (6.0 × 103) | L (5.0 × 103) K (5.0 × 104) | ||
| 34 | Triterpene |  | 3-keto-urs-11-en-13β(28)-olide(H27) | Unknown | 16.526 | C30H44O3 | 453.3396 | −7.25 | U (3.0 × 104) F (6.0 × 105) | U (2.0 × 104) K (1.4 × 105) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, M.-T.; Gong, M.-D.; Ma, X.; Xu, W.-B.; Wang, Z.-Y.; Tang, M.-H.; Wu, Y.; Liu, Z.-Y. Sex Differences in the In Vivo Exposure Process of Multiple Components of Gelsemium elegans in Rats. Metabolites 2023, 13, 33. https://doi.org/10.3390/metabo13010033
Zuo M-T, Gong M-D, Ma X, Xu W-B, Wang Z-Y, Tang M-H, Wu Y, Liu Z-Y. Sex Differences in the In Vivo Exposure Process of Multiple Components of Gelsemium elegans in Rats. Metabolites. 2023; 13(1):33. https://doi.org/10.3390/metabo13010033
Chicago/Turabian StyleZuo, Meng-Ting, Meng-Die Gong, Xiao Ma, Wen-Bo Xu, Zi-Yuan Wang, Mo-Huan Tang, Yong Wu, and Zhao-Ying Liu. 2023. "Sex Differences in the In Vivo Exposure Process of Multiple Components of Gelsemium elegans in Rats" Metabolites 13, no. 1: 33. https://doi.org/10.3390/metabo13010033