Bee Pollen and Probiotics May Alter Brain Neuropeptide Levels in a Rodent Model of Autism Spectrum Disorders
Abstract
:1. Introduction
2. Results and Discussion
Limitations
3. Materials and Methods
3.1. Materials
Prebiotic, Probiotic, and Fecal Transplant
3.2. Animals
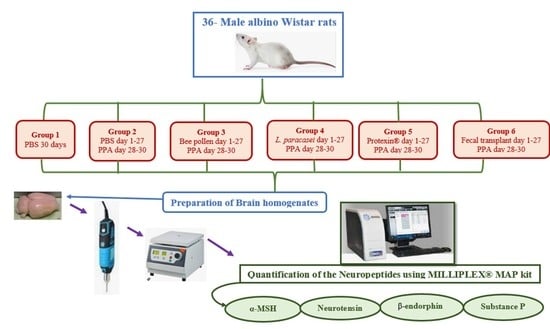
3.3. Study Design
3.4. Preparation of Brain Homogenate for the Identification of Neuropeptides
3.5. Quantification of the Neuropeptides in Brain Tissue
3.6. Statistical Analysis
3.6.1. Principal Component Analysis and Discriminant Analysis
3.6.2. Hierarchical Clustering
3.6.3. Receiver Operating Characteristic Curves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Al-Yousef, H.M.; Alkhulaifi, M.M.; Al-Salem, H.S.; Syed, R.A. Clostridium perfringens induced autism disorders counteract by using natural BP in vitro. J. Biol. Med. Res. 2018, 2, 8. [Google Scholar]
- Yan, Z.-X.; Gao, X.-J.; Li, T.; Wei, B.; Wang, P.-P.; Yang, Y.; Yan, R. Fecal Microbiota Transplantation in Experimental Ulcerative Colitis Reveals Associated Gut Microbial and Host Metabolic Reprogramming. Appl. Environ. Microbiol. 2018, 84, e00434e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, A.; Geurts, L.; Hoyles, L.; Iozzo, P.; Kraneveld, A.D.; La Fata, G.; Miani, M.; Patterson, E.; Pot, B.; Shortt, C.; et al. The microbiota–gut–brain axis: Pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell. Mol. Life Sci. 2022, 79, 80. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhao, F. Microbiota-gut-brain axis in autism spectrum disorder. J Genet. Genom. 2021, 48, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, H.; Cheng, Y.; Xu, F.; Ruan, G.; Ying, S.; Tang, W.; Chen, L.; Chen, M.; Lv, L.; et al. Fecal Microbiota Transplantation Relieves Gastrointestinal and Autism Symptoms by Improving the Gut Microbiota in an Open-Label Study. Front. Cell. Infect. Microbiol. 2021, 11, 759435. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, L.P.; Gandía, J.; Laboute, T.; Becker, J.A.J.; Le Merrer, J. μ opioid receptor, social behaviour and autism spectrum disorder: Reward matters. Br. J. Pharmacol. 2018, 175, 2750–2769. [Google Scholar] [CrossRef]
- Sandman, C.A.; Kastin, A.J. The influence of fragments of the LPH chains on learning, memory and attention in animals and man. Pharmacol. Ther. 1981, 13, 39–60. [Google Scholar] [CrossRef] [Green Version]
- Sahley, T.L.; Panksepp, J. Brain opioids and autism: An updated analysis of possible linkages. J. Autism Dev. Disord. 1987, 17, 201–216. [Google Scholar] [CrossRef]
- Harno, E.; Gali Ramamoorthy, T.; Coll, A.P.; White, A. HarnPOMC: The Physiological Power of Hormone Processing. Physiol. Rev. 2018, 98, 2381–2430. [Google Scholar] [CrossRef]
- Guastella, A.J.; Einfeld, S.L.; Gray, K.; Rinehart, N.; Tonge, B.; Lambert, T.J.; Hickie, I.B. Intranasal Oxytocin Improves Emotion Recognition for Youth with Autism Spectrum Disorders. Biol. Psychiatry 2010, 67, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Andari, E.; Duhamel, J.-R.; Zalla, T.; Herbrecht, E.; Leboyer, M.; Sirigu, A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc. Natl. Acad. Sci. USA 2010, 107, 4389–4394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzing, W.C.; Mufson, E.J.; Jennes, L.; Armstrong, D.M. Reduction of neurotensin immunoreactivity in the amygdala in Alzheimer’s disease. Brain Res. 1990, 537, 298–302. [Google Scholar] [CrossRef]
- Ferraro, L.; Beggiato, S.; Tomasini, M.C.; Fuxe, K.; Tanganelli, S.; Antonelli, T. Neurotensin regulates cortical inflammation transmission by modulating N-methyl-D-aspartate receptor functional activity: An in vivo microdialysis study. J. Neurosci. Res. 2011, 89, 1618–1626. [Google Scholar] [CrossRef]
- Yin, H.H.; Adermark, L.; Lovinger, D.M. Neurotensin reduces glutamatergic transmission in the dorsolateral striatum via retrograde endocannabinoid signaling. Neuropharmacology 2008, 54, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, V.A.; Kawahara, H.; Vaughan, C.W. Neurotensin inhibition of GABAergic transmission via mGluR-induced endocannabinoid signalling in rat periaqueductal grey. J. Physiol. 2009, 587, 2511–2520. [Google Scholar] [CrossRef]
- Anderson, E.J.P.; Çakir, I.; Carrington, S.J.; Cone, R.D.; Ghamari-Langroudi, M.; Gillyard, T.; Gimenez, L.E.; Litt, M.J. 60 Years of Pomc: Regulation of feeding and energy homeostasis by α-MSH. J. Mol. Endocrinol. 2016, 56, T157–T174. [Google Scholar] [CrossRef] [Green Version]
- Shamay-Tsoory, S.; Abu-Akel, A. Review: The social salience hypothesis of oxytocin. Biol. Psychiatry 2016, 79, 194–202. [Google Scholar] [CrossRef]
- Guastella, A.J.; Gray, K.M.; Rinehart, N.J.; Alvares, G.A.; Tonge, B.J.; Hickie, I.B.; Keating, C.M.; Cacciotti-Saija, C.; Einfeld, S.L. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: A randomized controlled trial. J. Child Psychol. Psychiatry 2015, 56, 444–452. [Google Scholar] [CrossRef]
- Dadds, M.R.; MacDonald, E.; Cauchi, A.; Williams, K.; Levy, F.; Brennan, J. Nasal oxytocin for social deficits in childhood autism: A randomized controlled trial. J. Autism Dev. Disord. 2014, 44, 521–531. [Google Scholar] [CrossRef]
- Ferrier, I.N.; Cross, A.J.; Johnson, J.A.; Roberts, G.W.; Crow, T.J.; Corsellis, J.A.; Lee, Y.C.; O’Shaughnessy, D.; Adrian, T.E.; McGregor, G.P.; et al. Neuropeptides in Alzheimer type dementia. J. Neurol. Sci. 1983, 62, 159–170. [Google Scholar] [CrossRef]
- Kyriatzis, G.; Bernard, A.; Bôle, A.; Pflieger, G.; Chalas, P.; Masse, M.; Lécorché, P.; Jacquot, G.; Ferhat, L.; Khrestchatisky, M. Neurotensin receptor 2 is induced in astrocytes and brain endothelial cells in relation to neuroinflammation following pilocarpine-induced seizures in rats. GLIA 2021, 69, 2618–2643. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A. GABA and Glutamate Imbalance in Autism and Their Reversal as Novel Hypothesis for Effective Treatment Strategy. Autism Dev. Disord. 2020, 18, 46–63. [Google Scholar] [CrossRef]
- Caceda, R.; Kinkead, B.; Nemeroff, C.B. Neurotensin: Role in psychiatric and neurological diseases. Peptides 2006, 27, 2385–2404. [Google Scholar] [CrossRef]
- Drew, G.M.; Mitchell, V.A.; Vaughan, C.W. Glutamate spillover modulates GABAergic synaptic transmission in the rat midbrain periaqueductal grey via metabotropic glutamate receptors and endocannabinoid signaling. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 808–815. [Google Scholar] [CrossRef]
- Petkova-Kirova, P.; Rakovska, A.; Della Corte, L.; Zaekova, G.; Radomirov, R.; Mayer, A. Neurotensin modulation of acetylcholine, GABA, and aspartate release from rat prefrontal cortex studied in vivo with microdialysis. Brain Res. Bull. 2008, 77, 129–135. [Google Scholar] [CrossRef]
- Petrie, K.A.; Schmidt, D.; Bubser, M.; Fadel, J.; Carraway, R.E.; Deutch, A.Y. Neurotensin activates GABAergic interneurons in the prefrontal cortex. J. Neurosci. 2005, 25, 1629–1636. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Geiger, J.D.; Lei, S. Neurotensin enhances GABAergic activity in rat hippocampus CA1 region by modulating L-type calcium channels. J. Neurophysiol. 2008, 99, 2134–2143. [Google Scholar] [CrossRef] [Green Version]
- Boules, M.; Cusack, B.; Zhao, L.; Fauq, A.; McCormick, D.J.; Richelson, E. A novel neurotensin peptide analog given extracranially decreases food intake and weight in rodents. Brain Res. 2000, 865, 35–44. [Google Scholar] [CrossRef]
- Feifel, D.; Goldenberg, J.; Melendez, G.; Shilling, P.D. The acute and subchronic effects of a brain-penetrating, neurotensin-1 receptor agonist on feeding, body weight and temperature. Neuropharmacology 2010, 58, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Boules, M.; Williams, K.; Gordillo, A.; Li, S.; Richelson, E. Similarities in the behavior and molecular deficits in the frontal cortex between the neurotensin receptor subtype 1 knockout mice and chronic phencyclidine-treated mice: Relevance to schizophrenia. Neurobiol. Dis. 2010, 40, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.Y.; Du, Y.F.; Chen, L. Neuropeptides exert neuroprotective effects in Alzheimer’s disease. Front. Mol. Neurosci. 2019, 11, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stumm, R.; Culmsee, C.; Schafer, M.K.; Krieglstein, J.; Weihe, E. Adaptive plasticity in tachykinin and tachykinin receptor expression after focal cerebral ischemia is differentially linked to gabaergic and glutamatergic cerebrocortical circuits and cerebrovenular endothelium. J. Neurosci. 2001, 21, 798–811. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S.; Wass, C.A.; Cross, A.S.; Opal, S.M. Modulation of blood–brain barrier permeability by tumor necrosis factor and antibody to tumor necrosis factor in the rat. Lymphokine Cytokine Res. 1992, 11, 293–298. [Google Scholar]
- Theoharides, T.C.; Doyle, R. Autism, gut-blood–brain barrier and mast cells. J. Clin. Psychopharm. 2008, 28, 479–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejeda, H.A.; Shippenberg, T.S.; Henriksson, R. The dynorphin/κ-opioid receptor system and its role in psychiatric disorders. Cell. Mol. Life Sci. 2012, 69, 857–896. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Matsumoto, K.; Tohda, M.; Kaneko, Y.; Watanabe, H. Diazepambinding inhibitor (DBI) gene expression in the brains of socially isolated and group-housed mice. Neurosci. Res. 1999, 33, 171–177. [Google Scholar] [CrossRef]
- Kokare, D.M.; Dandekar, M.P.; Singru, P.S.; Gupta, G.L.; Subhedar, N.K. Involvement of alpha-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology 2010, 58, 1009–1018. [Google Scholar] [CrossRef]
- Bandelow, B.; Wedekind, D. Possible role of a dysregulation of the endogenous opioid system in antisocial personality disorder. Hum. Psychopharmacol. 2015, 30, 393–415. [Google Scholar] [CrossRef]
- Hang, A.; Wang, Y.J.; He, L.; Liu, J.G. The role of the dynorphin/κ opioid receptor system in anxiety. Acta Pharmacol. Sin. 2015, 36, 783–790. [Google Scholar] [CrossRef]
- Fujii, T.; Hattori, K.; Miyakawa, T.; Ohashi, Y.; Sato, H.; Kunugi, H. Metabolic profile alterations in the postmortem brains of patients with schizophrenia using capillary electrophoresis-mass spectrometry. Schizophr. Res. 2017, 183, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.G.; Kuddo, T.; Song, E.Y.; Dambrosia, J.M.; Kohler, S.; Satyanarayana, G.; VanDunk, C.; Grether, J.K.; Nelson, K.B. Selected neurotrophins, neuropeptides, and cytokines: Developmental trajectory and concentrations in neonatal blood of children with autism or Down syndrome. Int. J. Dev. Neurosci. 2006, 24, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Keiser, A.A.; Matazel, K.S.; Esser, M.K.; Feifel, D.; Prus, A.J. Systemic administration of the neurotensin NTS₁-receptor agonist PD149163 improves performance on a memory task in naturally deficient male brown Norway rats. Exp. Clin. Psychopharmacol. 2014, 22, 541–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.; Cilz, N.I.; Kurada, L.; Hu, B.; Yang, C.; Wada, E.; Combs, C.K.; Porter, J.E.; Lesage, F.; Lei, S. Activation of neurotensin receptor 1 facilitates neuronal excitability and spatial learning and memory in the entorhinal cortex: Beneficial actions in an Alzheimer’s disease model. J. Neurosci. 2014, 34, 7027–7042. [Google Scholar] [CrossRef] [Green Version]
- Fetissov, S.O.; Averina, O.V.; Danilenko, V.N. Neuropeptides in the microbiota-brain axis and feeding behavior in autism spectrum disorder. Nutrition 2019, 61, 43–48. [Google Scholar] [CrossRef]
- Tsilioni, I.; Patel, A.B.; Pantazopoulos, H.; Berretta, S.; Conti, P.; Leeman, S.E.; Theoharides, T.C. IL-37 is increased in brains of children with autism spectrum disorder and inhibits human microglia stimulated by neurotensin. Proc. Natl. Acad. Sci. USA 2019, 116, 21659–21665. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, G.A.; Al-Ayadhi, L.Y. The possible link between the elevated serum levels of neurokinin A and anti-ribosomal P protein antibodies in children with autism. J. Neuroinflamm. 2011, 8, 180. [Google Scholar] [CrossRef] [Green Version]
- Tungland, B. Gut microbiota in brain development and disorders of the cns: Therapeutic strategies involving dietary modification, pro- and prebiotic intervention, and fecal microbiota transplantation (fmt) therapy. In Human Microbiota in Health and Disease; Tungland, B., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 517–594. [Google Scholar]
- Stewart, S.; Ivy, M.A.; Anslyn, E.V. The use of principal component analysis and discriminant analysis in differential sensing routines. Chem. Soc. Rev. 2013, 43, 70–84. [Google Scholar] [CrossRef]
- Kaiser, H.F. A note on the equamax criterion. Multivar. Behav. Res. 1974, 9, 501–503. [Google Scholar] [CrossRef]
- Tomlinson, A.; Hair, M.; McFadyen, A. Statistical approaches to assessing single and multiple outcome measures in dry eye therapy and diagnosis. Ocul Surf. 2013, 11, 267–284. [Google Scholar] [CrossRef]
- Bartlett, M. Properties of sufficiency and statistical tests. Proc. R. Soc. London Ser. A-Math. Phys. Sci. 1937, 160, 268–282. [Google Scholar]
- O’connor, B.P. SPSS and SAS programs for determining the number of components using parallel analysis and Velicer’s MAP test. Behav. Res. Methods Instrum. Comput. 2000, 32, 396–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perlis, R.H. Translating biomarkers to clinical practice. Mol. Psychiatry 2011, 16, 1076–1087. [Google Scholar] [CrossRef] [PubMed]



| All Groups | PPA and Control | ||||||
|---|---|---|---|---|---|---|---|
| PC1 (80.81%) | PC2 (11.15%) | PC1 (55.23%) | PC2 (27.83%) | ||||
| α-MSH | 5.697 | β-End | 3.414 | SP | 3.168 | NT | 2.802 |
| SP | 5.650 | NT | −1.940 | α-MSH | 2.742 | β-End | −1.960 |
| NT | 5.307 | SP | −0.619 | β-End | 2.569 | α-MSH | 1.083 |
| β-End | 4.879 | α-MSH | −0.504 | NT | 1.535 | SP | −0.706 |
| All Groups | PPA and Control | ||||
|---|---|---|---|---|---|
| PC1 (97.54%) | PC2 (1.52%) | PC1 (100%) | |||
| α-MSH | −2.784 | NT | 0.374 | α-MSH | −1.592 |
| NT | −2.422 | α-MSH | −0.340 | SP | −1.032 |
| SP | −2.326 | β-End | 0.239 | NT | −0.782 |
| β-End | −1.515 | SP | −0.138 | β-End | −0.751 |
| ROC Analysis | AUC | p Value | Cutoff | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| PCA | 0.889 | 0.025 | −1.45 | 100 | 83.3 |
| DA | 0.889 | 0.025 | 0.35 | 100 | 83.3 |
| α-MSH | 0.833 | 0.055 | 301 | 83.3 | 83.3 |
| β-End | 0.861 | 0.037 | 1965 | 100 | 83.3 |
| NT | 0.778 | 0.109 | 645 | 83.3 | 50.0 |
| SP | 0.806 | 0.078 | 52 | 83.3 | 83.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, M.A.; Al-Ayadhi, L.; Hassan, W.M.; Bhat, R.S.; Alonazi, M.A.; El-Ansary, A. Bee Pollen and Probiotics May Alter Brain Neuropeptide Levels in a Rodent Model of Autism Spectrum Disorders. Metabolites 2022, 12, 562. https://doi.org/10.3390/metabo12060562
Alghamdi MA, Al-Ayadhi L, Hassan WM, Bhat RS, Alonazi MA, El-Ansary A. Bee Pollen and Probiotics May Alter Brain Neuropeptide Levels in a Rodent Model of Autism Spectrum Disorders. Metabolites. 2022; 12(6):562. https://doi.org/10.3390/metabo12060562
Chicago/Turabian StyleAlghamdi, Mashael A., Laila Al-Ayadhi, Wail M. Hassan, Ramesa Shafi Bhat, Mona A. Alonazi, and Afaf El-Ansary. 2022. "Bee Pollen and Probiotics May Alter Brain Neuropeptide Levels in a Rodent Model of Autism Spectrum Disorders" Metabolites 12, no. 6: 562. https://doi.org/10.3390/metabo12060562