Production of Hydroxy Fatty Acids, Precursors of γ-Hexalactone, Contributes to the Characteristic Sweet Aroma of Beef
Abstract
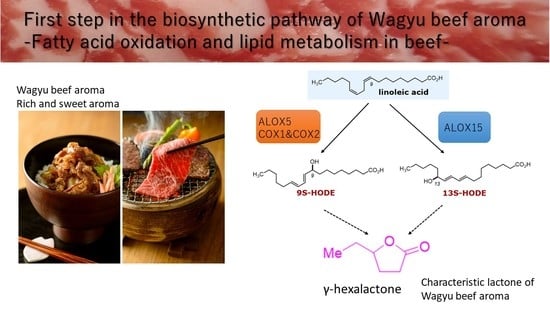
:1. Introduction
2. Results
2.1. Preparation of Beef Tallow by Vacuum Distillation
2.2. Quantification of Odorants by Dynamic Headspace–Gas Chromatography–Mass Spectroscopy
2.3. Analysis of Hydroxylated Metabolites by Liquid Chromatography–Mass Spectrometry
2.4. Expression Analysis of Linoleic-Acid-Related Oxidase in Muscle and Fat Tissues
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Purification of Beef Tallow by Vacuum Extraction
4.3. Analysis of Fatty Acids Composition
4.4. Analysis of Odor Components of Beef Tallow after Heating
4.5. Sensory Evaluation
4.6. LC-MS/MS Analysis of Hydroxy Fatty Acids
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LC-MS/MS | liquid chromatography coupled with a triple quadrupole mass spectrometer system |
| 9-HpODE | 9-hydroperoxy-11,12-octadecadienoic acid |
| 13-HpODE | 13-hydroperoxy-(9Z,11E)-octadecadienoic acid |
| 9-KODE | 9-oxo-octadecadienoic acid |
| 13-KODE | 13-oxo-octadecadienoic acid |
| 9S-HODE | 9- hydroxyoctadecadienoic acid |
| 13S-HODE | 13-hydroxyoctadecadienoic acid |
| 9(10)-EpOME | 9,10-epoxy-12-octadecenoic acid |
| 12(13)-EpOME | 12,13-epoxy-9-octadecenoic acid |
| 9(10)-diHOME | 9,10-dihydroxyoctadec-12-enoic acid |
| 12(13)-diHOME | 12,13-dihydroxyoctadec-9-enoic acid |
| ALOX15 | arachidonate 15-lipoxygenase |
| ALOX15B | arachidonate 15-lipoxygenase type B |
| COX1 | cyclooxygenases 1 |
| COX2 | cyclooxygenases 2 |
| ALOX5 | arachidonate 5-lipoxygenase |
References
- Kaewpila, C.; Sommart, K.; Mitsumori, M. Dietary fat sources affect feed intake, digestibility, rumen microbial populations, energy partition and methane emissions in different beef cattle genotypes. Animal 2018, 12, 2529–2538. [Google Scholar] [CrossRef] [PubMed]
- Soice, E.; Johnston, J. Immortalizing Cells for Human Consumption. Int. J. Mol. Sci. 2021, 22, 11660. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczak, K.; Onopiuk, A.; Szpicer, A.; Poltorak, A. Meat Analogues in the Perspective of Recent Scientific Research: A Review. Foods 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Grossmann, L. A brief review of the science behind the design of healthy and sustainable plant-based foods. NPJ Sci. Food 2021, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Aaslyng, M.D.; Meinert, L. Meat flavour in pork and beef—From animal to meal. Meat Sci. 2017, 132, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chem. 2022, 371, 131139. [Google Scholar] [CrossRef]
- Motoyama, M.; Sasaki, K.; Watanabe, A. Wagyu and the factors contributing to its beef quality: A Japanese industry overview. Meat Sci. 2016, 120, 10–18. [Google Scholar] [CrossRef]
- Ueda, S.; Hosoda, M.; Yoshino, K.-i.; Yamanoue, M.; Shirai, Y. Gene Expression Analysis Provides New Insights into the Mechanism of Intramuscular Fat Formation in Japanese Black Cattle. Genes 2021, 12, 1107. [Google Scholar] [CrossRef]
- Gotoh, T.; Nishimura, T.; Kuchida, K.; Mannen, H. The Japanese Wagyu beef industry: Current situation and future prospects —A review. Asian-Australas. J. Anim. Sci. 2018, 31, 933–950. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.V.; Nguyen, O.C.; Malau-Aduli, A.E.O. Main regulatory factors of marbling level in beef cattle. Vet. Anim. Sci. 2021, 14, 100219. [Google Scholar] [CrossRef]
- Inagaki, S.; Amano, Y.; Kumazawa, K. Identification and Characterization of Volatile Components Causing the Characteristic Flavor of Wagyu Beef (Japanese Black Cattle). J. Agric. Food Chem. 2017, 65, 8691–8695. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Tago, A.; Yoshinaga-Kiriake, A.; Gotoh, N. Characterization of lactones in Wagyu (Japanese beef) and imported beef by combining solvent extraction and gas chromatography–mass spectrometry. LWT 2021, 135, 110015. [Google Scholar] [CrossRef]
- Matsuishi, M.; Fujimori, M.; Okitani, A. Wagyu Beef Aroma in Wagyu (Japanese Black Cattle) Beef Preferred by the Japanese over Imported Beef. Nihon Chikusan Gakkaiho 2001, 72, 498–504. [Google Scholar] [CrossRef]
- Ueda, S.; Yamanoue, M.; Sirai, Y.; Iwamoto, E. Exploring the Characteristic Aroma of Beef from Japanese Black Cattle (Japanese Wagyu) via Sensory Evaluation and Gas Chromatography-Olfactometry. Metabolites 2021, 11, 56. [Google Scholar] [CrossRef]
- Kang, W.R.; Seo, M.J.; An, J.U.; Shin, K.C.; Oh, D.K. Production of δ-decalactone from linoleic acid via 13-hydroxy-9(Z)-octadecenoic acid intermediate by one-pot reaction using linoleate 13-hydratase and whole Yarrowia lipolytica cells. Biotechnol. Lett. 2016, 38, 817–823. [Google Scholar] [CrossRef]
- Ueda, S.; Sasaki, R.; Nakabayashi, R.; Yamanoue, M.; Sirai, Y.; Iwamoto, E. Exploring the Lipids Involved in the Formation of Characteristic Lactones in Japanese Black Cattle. Metabolites 2021, 11, 203. [Google Scholar] [CrossRef]
- Kishimoto, T.; Noba, S.; Yako, N.; Kobayashi, M.; Watanabe, T. Simulation of Pilsner-type beer aroma using 76 odor-active compounds. J. Biosci. Bioeng. 2018, 126, 330–338. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Zhang, B.; Shen, C.; Xu, Y.; Tang, K. Identification, quantitation and sensorial contribution of lactones in brandies between China and France. Food Chem. 2021, 357, 129761. [Google Scholar] [CrossRef]
- Leonardou, V.K.; Doudoumis, E.; Tsormpatsidis, E.; Vysini, E.; Papanikolopoulos, T.; Papasotiropoulos, V.; Lamari, F.N. Quality Traits, Volatile Organic Compounds, and Expression of Key Flavor Genes in Strawberry Genotypes over Harvest Period. Int. J. Mol. Sci. 2021, 22, 13499. [Google Scholar] [CrossRef]
- Watkins, P.J.; Rose, G.; Warner, R.D.; Dunshea, F.R.; Pethick, D.W. A comparison of solid-phase microextraction (SPME) with simultaneous distillation–extraction (SDE) for the analysis of volatile compounds in heated beef and sheep fats. Meat Sci. 2012, 91, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Kerth, C. Determination of volatile aroma compounds in beef using differences in steak thickness and cook surface temperature. Meat Sci. 2016, 117, 27–35. [Google Scholar] [CrossRef]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Influence of thermal treatment on formation of volatile compounds, cooking loss and lipid oxidation in foal meat. LWT Food Sci. Technol. 2014, 58, 439–445. [Google Scholar] [CrossRef]
- Wanikawa, A.; Hosoi, K.; Kato, T. Conversion of Unsaturated Fatty Acids to Precursors of γ-Lactones by Lactic Acid Bacteria during the Production of Malt Whisky. J. Am. Soc. Brew. Chem. 2000, 58, 51–56. [Google Scholar] [CrossRef]
- Andres Contreras, G.; De Koster, J.; de Souza, J.; Laguna, J.; Mavangira, V.; Nelli, R.K.; Gandy, J.; Lock, A.L.; Sordillo, L.M. Lipolysis modulates the biosynthesis of inflammatory lipid mediators derived from linoleic acid in adipose tissue of periparturient dairy cows. J. Dairy Sci. 2020, 103, 1944–1955. [Google Scholar] [CrossRef]
- Kaduce, T.L.; Figard, P.H.; Leifur, R.; Spector, A.A. Formation of 9-hydroxyoctadecadienoic acid from linoleic acid in endothelial cells. J. Biol. Chem. 1989, 264, 6823–6830. [Google Scholar] [CrossRef]
- Orafaie, A.; Matin, M.M.; Sadeghian, H. The importance of 15-lipoxygenase inhibitors in cancer treatment. Cancer Metastasis Rev. 2018, 37, 397–408. [Google Scholar] [CrossRef]
- Rådmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 331–339. [Google Scholar] [CrossRef]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.-S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef]
- Ueda, S.; Iwamoto, E.; Kato, Y.; Shinohara, M.; Shirai, Y.; Yamanoue, M. Comparative metabolomics of Japanese Black cattle beef and other meats using gas chromatography-mass spectrometry. Biosci. Biotechnol. Biochem. 2019, 83, 137–147. [Google Scholar] [CrossRef]
- Shirouchi, B.; Albrecht, E.; Nuernberg, G.; Maak, S.; Olavanh, S.; Nakamura, Y.; Sato, M.; Gotoh, T.; Nuernberg, K. Fatty acid profiles and adipogenic gene expression of various fat depots in Japanese Black and Holstein steers. Meat Sci. 2014, 96, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S.; Edwards, R.A. The role of triglycerides and phospholipids in the aroma of cooked beef. J. Sci. Food Agric. 1983, 34, 517–522. [Google Scholar] [CrossRef]
- Tikk, K.; Tikk, M.; Aaslyng, M.D.; Karlsson, A.H.; Lindahl, G.; Andersen, H.J. Significance of fat supplemented diets on pork quality—Connections between specific fatty acids and sensory attributes of pork. Meat Sci. 2007, 77, 275–286. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleh, A.S.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef]
- Celia Resconi, V.; del Mar Campo, M.; Montossi, F.; Ferreira, V.; Sañudo, C.; Escudero, A. Gas Chromatographic-Olfactometric Aroma Profile and Quantitative Analysis of Volatile Carbonyls of Grilled Beef from Different Finishing Feed Systems. J. Food Sci. 2012, 77, S240–S246. [Google Scholar] [CrossRef]
- Hu, H.; Shi, A.; Liu, H.; Liu, L.; Fauconnier, M.L.; Wang, Q. Study on Key Aroma Compounds and Its Precursors of Peanut Oil Prepared with Normal- and High-Oleic Peanuts. Foods 2021, 10, 3036. [Google Scholar] [CrossRef]
- Utama, D.T.; Lee, S.G.; Baek, K.H.; Jang, A.; Pak, J.I.; Lee, S.K. Effects of high-pressure processing on taste-related ATP breakdown compounds and aroma volatiles in grass-fed beef during vacuum aging. Asian–Australas. J. Anim. Sci. 2018, 31, 1336–1344. [Google Scholar] [CrossRef]
- Migita, K.; Iiduka, T.; Tsukamoto, K.; Sugiura, S.; Tanaka, G.; Sakamaki, G.; Yamamoto, Y.; Takeshige, Y.; Miyazawa, T.; Kojima, A.; et al. Retort beef aroma that gives preferable properties to canned beef products and its aroma components. Anim. Sci. J. 2017, 88, 2050–2056. [Google Scholar] [CrossRef]
- Faccia, M. The Flavor of Dairy Products from Grass-Fed Cows. Foods 2020, 9, 1188. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, Z.; Emami, S.; Chen, J.; Leite Nobrega de Moura Bell, J.M.; Taha, A.Y. Triacylglycerols are preferentially oxidized over free fatty acids in heated soybean oil. NPJ Sci. Food 2021, 5, 7. [Google Scholar] [CrossRef]
- Yang, Z.; Piironen, V.; Lampi, A.-M. Lipid-modifying enzymes in oat and faba bean. Food Res. Int. 2017, 100, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Cabral, M.; Martín-Venegas, R.; Moreno, J.J. Differential cell growth/apoptosis behavior of 13-hydroxyoctadecadienoic acid enantiomers in a colorectal cancer cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G664–G671. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, I.; Kuhn, H.; Heydeck, D. Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15). Gene 2015, 573, 1–32. [Google Scholar] [CrossRef]
- Jain, R.; Austin Pickens, C.; Fenton, J.I. The role of the lipidome in obesity-mediated colon cancer risk. J. Nutr. Biochem. 2018, 59, 1–9. [Google Scholar] [CrossRef]
- Hijioka, M.; Futokoro, R.; Ohto-Nakanishi, T.; Nakanishi, H.; Katsuki, H.; Kitamura, Y. Microglia-released leukotriene B4 promotes neutrophil infiltration and microglial activation following intracerebral hemorrhage. Int. Immunopharmacol. 2020, 85, 106678. [Google Scholar] [CrossRef]
- Ueda, S.; Kokaji, Y.; Simizu, S.; Honda, K.; Yoshino, K.-i.; Kamisoyama, H.; Shirai, Y.; Yamanoue, M. Chicken heat shock protein HSPB1 increases and interacts with αB-crystallin in aged skeletal muscle. Biosci. Biotechnol. Biochem. 2015, 79, 1867–1875. [Google Scholar] [CrossRef] [Green Version]






| Sample Names | Tissue | Species | Vacuum Distillation (%) | ||
|---|---|---|---|---|---|
| Beef Tallow | Solid Content | Evaporated Water | |||
| Fats | Intermuscular fats | Japanese Black cattle | 56.8 | 29.2 | 14.1 |
| Marble | Muscle and intramuscular fats | Japanese Black cattle | 35.5 | 33.3 | 31.2 |
| Muscle | Muscle | Holstein cattle | 12.8 | 34.6 | 52.7 |
| Metabolite | Japanese Black | Hostein Muscle | |
|---|---|---|---|
| Fats | Marble | ||
| 9-HpODE a | 42.0 | 30.7 | 66.7 |
| 13-HpODE | - | - | - |
| 9-HODE | 269.9 | 310.8 | 382.7 |
| 13-HODE | 361.0 | 361.9 * | 518.5 ** |
| 9-KODE | 51.1 | 57.6 | 111.3 |
| 13-KODE | 78.9 | 79.4 | 118.8 |
| 9-EpOME a | 29.1 | 33.6 | 47.6 |
| 12-EpOME | 70.4 | 74.5 | 84.2 |
| 9-diHOME | 1.83 | 1.43 | 2.21 |
| 12-diHOME | 69.7 | 53.6 | 71.4 |
| Quantitative value (pg/mg beef tallow) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, S.; Hosoda, M.; Kasamatsu, K.; Horiuchi, M.; Nakabayashi, R.; Kang, B.; Shinohara, M.; Nakanishi, H.; Ohto-Nakanishi, T.; Yamanoue, M.; et al. Production of Hydroxy Fatty Acids, Precursors of γ-Hexalactone, Contributes to the Characteristic Sweet Aroma of Beef. Metabolites 2022, 12, 332. https://doi.org/10.3390/metabo12040332
Ueda S, Hosoda M, Kasamatsu K, Horiuchi M, Nakabayashi R, Kang B, Shinohara M, Nakanishi H, Ohto-Nakanishi T, Yamanoue M, et al. Production of Hydroxy Fatty Acids, Precursors of γ-Hexalactone, Contributes to the Characteristic Sweet Aroma of Beef. Metabolites. 2022; 12(4):332. https://doi.org/10.3390/metabo12040332
Chicago/Turabian StyleUeda, Shuji, Mana Hosoda, Kumi Kasamatsu, Masahiro Horiuchi, Rio Nakabayashi, Bubwoong Kang, Masakazu Shinohara, Hiroki Nakanishi, Takayo Ohto-Nakanishi, Minoru Yamanoue, and et al. 2022. "Production of Hydroxy Fatty Acids, Precursors of γ-Hexalactone, Contributes to the Characteristic Sweet Aroma of Beef" Metabolites 12, no. 4: 332. https://doi.org/10.3390/metabo12040332