Functional Nutrients to Ameliorate Neurogenic Muscle Atrophy
Abstract
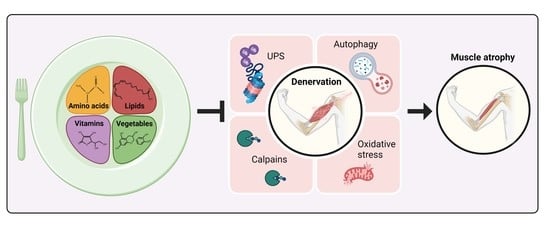
:1. Neurogenic Muscle Atrophy
1.1. Pathways Triggered by Denervation in Skeletal Muscle
1.1.1. Ubiquitin-Proteasome System
1.1.2. Autophagy
1.1.3. Calpains
1.1.4. Oxidative Stress
1.1.5. AKT-mTOR
2. Functional Foods to Ameliorate Neurogenic Muscle Atrophy
2.1. Protein or Amino Acids
- Branched-chain amino acids (BCAAs), i.e., leucine, isoleucine, and valine, are three of the nine essential amino acids and are found in protein-rich foods such as eggs, meat, and dairy products. A BCAA-enriched diet has been shown to be protective against sarcopenia in both slow and fast muscles of old mice [94] and in elderly subjects [89,95,96], by promoting mitochondrial genesis, thus improving muscle endurance, and decreasing oxidative stress. In contrast, treatment of ALS patients with BCAAs or L-threonine for six months failed to show beneficial effects on disease progression [97].
- Leucine is an essential BCAA present in all protein-rich foods, but is more abundant in those of animal origin [98]. A leucine-enriched exclusive diet prevented neurogenic muscle atrophy in denervated rat soleus (slow) muscle, by increasing the AKT/mTOR anabolic pathway and decreasing the AMPK-catabolic one [99]. Leucine supplementation is potentially useful to increase protein synthesis for counteracting muscle sarcopenia in elderly subjects [100,101].
- Beta-Hydroxy-Beta-Methyl Butyrate (HMB) is a natural metabolite of the amino acid leucine, and is found in small quantities in grapefruit, alfalfa, and catfish. In humans, 2–10% of dietary L-leucine is converted to HMB, corresponding to about 0.3 g/day; however, when taken as a supplement the doses are 10–20-fold higher [104]. As a derivate of a BCAA, HMB is thought to act as pro-anabolic and anti-catabolic compound for skeletal muscle, and early studies showed a positive impact of HMB supplementation in counteracting the age-related losses of skeletal muscle mass in elderly subjects [105,106,107]. However, two recent articles published opposite results: according to a review, the current evidence is inconclusive with regard to any positive effects of HMB supplementation on functional outcome measurements or muscle mass in elderly human subjects and in hospitalized patients [108], while a meta-analysis concluded that HMB supplementation helps increase muscle strength in elderly people [109]. To the best of our knowledge, no studies on HMB supplementation in animal models of neurogenic muscle atrophy are currently available. Studies are needed to clarify the pathways hit by HMB in denervated or aged skeletal muscles, prior to proposing the use of HMB for counteracting neurogenic muscle atrophy or sarcopenia.
- Creatine is a compound derived from glycine and arginine which is mostly present in skeletal muscle, where it is used as an energy store, and can be found in red meat and seafood. Abundant evidence indicates that creatine supplementation increases skeletal muscle mass and strength if associated with resistance training [110], due to its beneficial effects on decreasing muscle protein breakdown, inflammation, and oxidative stress; interestingly, this also holds true with aging [111,112]. Despite the positive effects on skeletal muscle homeostasis, current literature suggests that exogenous creatine supplementation appears to be poorly effective in treating ALS [113,114].
- Carnitine is a quaternary ammonium compound required for the transport of long-chain fatty acids into mitochondria for energy production. Carnitine can be mainly found in animal products such as meat, fish, poultry, and milk, and is involved in skeletal muscle protein homeostasis by regulating both protein synthesis and breakdown, being an antioxidant and anti-inflammatory compound [115]. Interestingly, denervation and aging decrease carnitine levels in both slow and fast rat skeletal muscles [116], suggesting a causative role for carnitine in the atrophic program. Carnitine supplementation, alone or in combination with physical exercise, counteracts the age-dependent decline of mitochondrial function in the soleus rat muscle, improving muscle energy production and body protein mass [117,118,119]. The positive effects of carnitine supplementation were also reported in a murine model of ALS [120], as well as in a phase II clinical trial [121], proving to be effective in slowing down the progression of muscle weakness and prolonging mouse and patient survival.
- Carnosine is a dipeptide composed of the beta-alanine and histidine amino acids and mainly present in meats. Carnosine exerts numerous positive actions in skeletal muscle, including antioxidant and antiglycation activity, enhanced calcium sensitivity, and H+ buffering [122] that may affect muscle performance and maintenance in aging and neuromuscular diseases [123,124,125]. In elderly subjects, an increase in carnosine intake counteracted cognitive decline and improved physical capacity, probably due to its anti-inflammatory action [126,127]. Further studies are needed to clarify the effects of carnosine supplementation on skeletal muscle proteolysis and synthesis in a denervation-dependent condition such as in aging or ALS.
| Functional Nutrient | Disease or Condition | Experimental Model | Mechanism of Action | References |
|---|---|---|---|---|
| BCAAs | Sarcopenia | Aged mice | - Promote mitochondrial formation and function - Decrease oxidative stress | [94] |
| Elderly human subjects | [95,96,128] | |||
| Leucine | Nerve rescission | Rat | - Increases the AKT/mTOR - Decreases the AMPK catabolic pathways | [99] |
| Sarcopenia | Elderly human subjects | - Improves lean muscle-mass content | [100,101] | |
| Creatine | Sarcopenia | Adults human subjects | - Decreases muscle protein breakdown, inflammation, and oxidative stress | [110,111,112] |
| Carnitine | Sarcopenia | Rat | - Increases mitochondrial function and muscle mass | [117,118,119] |
| ALS | Mice and human patients | - Delays ALS onset and progression, prolongs survival | [120,121] | |
| Carnosine | Aging | Elderly human subjects | - Reduces OS, inflammation, inhibits protein glycation and aggregation | [126,127] |
2.2. Lipids
- Linoleic acid (LA) is an omega-6 PUFA that is found in vegetable oils, nuts, seeds, meats, and eggs [136]. LA was already known for its beneficial effects on skeletal muscle cells, demonstrated in vitro [137] and in vivo against muscular dystrophy [138]. Very recently, it has also been shown that LA treatment counteracts neurogenic muscle atrophy in mice by preventing the denervation-induced increase of oxidative stress and UPS [139].
- Fish oil is available from many types of fish and shellfish. It is rich in two important omega-3 fatty acids, i.e., eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). A fish oil-enriched diet has been shown to be protective against neurogenic muscle atrophy in mice by suppressing the TNF-α-dependent UPS catabolic pathway [140]. Interestingly, a therapy based on fish oil-derived n3 PUFA has been proposed as a natural approach in a clinical trial to counteract sarcopenia, being partially effective in preventing the decline of muscle volume and strength in elderly subjects [134]. Jeromson et al. [141] stress that n-3 supplementation in humans increases sensitivity to anabolic stimuli and shows additional, anticatabolic effects. While EPA has been shown to antagonize the actions of TNF-α on C2C12 myotube formation, it also diminishes the activation of the NF-κB pathway, thereby reducing MuRF1 signaling in these cells. EPA, in addition, favorably affects cell metabolism in the face of alterations in the availability of the different energy substrates, promoting plasticity, and improves glucose uptake in cultured myotubes, as reported in [141].
- Medium-chain triglycerides (MCT) are six-twelve carbon fatty acid esters of glycerol that can be found in coconut oil and products, palm oil, and dairy products. In mice, an MCT-enriched diet triggers glucose and lipid metabolism pathways and mitochondrial biogenesis in the skeletal muscle, thus improving muscle function under high-temperature conditions [143]. MCTs alone, or in combination with leucine and vitamin D, increase muscle strength and function in elderly adults [144,145]. However, MCT administration is detrimental for the heart of healthy or dystrophic mice [146,147]. Thus, careful examination of the systemic effects of MCT administration is encouraged in clinical studies.
- Alkylresorcinols (ARs), differently from PUFA, are amphiphilic phenolic lipids present in many kinds of cereals. Interestingly, AR dietary supplementation prevented neurogenic muscle atrophy in mice by affecting the autophagic pathway and thereby the lipid metabolism, but not the UPS activation [148]. This finding is intriguing, confirming that challenging metabolism may be an efficient therapeutic approach for maintaining muscle homeostasis, as suggested in ALS [149] and already described in other muscle pathologies [35,150,151].
| Functional Nutrient | Disease or Condition | Experimental Model | Mechanism of Action | References |
|---|---|---|---|---|
| Omega-3 fatty acids | Sarcopenia | Elderly human subjects | - Stimulates muscle protein synthesis via mTOR | [133] |
| Fish oil-derived omega-3 fatty acids | Sarcopenia | Elderly human subjects | - Increases muscle mass and function | [134] |
| Omega-3 fatty acids | ALS | ALS patients | - Prevents or delays ALS onset | [131] |
| Linoleic acid | Nerve Rescission | Mice | - Counteracts the increase of oxidative stress and UPS activation | [139] |
| Fish oil | Nerve Rescission | Mice | - Suppresses the UPS activation | [140] |
| MCTs | Elderly human subjects | - Increases muscle strength and function | [144] | |
| Alkylresorcinols | Nerve Rescission | Mice | - Modulates autophagy | [148] |
2.3. Vitamins
- β-carotene is a red-orange pigment found in fruits and vegetables. The human body converts β-carotene into vitamin A. β-carotene attenuates ROS-dependent muscle atrophy in C2C12 myotubes by repressing the activation of atrogin-1 and MuRF1 [153]. In the elderly, high plasma concentrations of β-carotene and other similar antioxidants correlate with preserved muscle function [154], which would support their clinical use. However, weak data were reported about the effectiveness against neurogenic muscle atrophy in vivo, as β-carotene supplementation is only effective at the early stage of soleus muscle atrophy upon denervation [155].
- As for vitamin C, both dietary and circulating levels positively correlate with skeletal muscle mass measurements in middle- and older-aged subjects, suggesting that dietary vitamin C intake may be protective against sarcopenia [156]. Coherently, mice with defective vitamin C biosynthesis (SMP30-knockout mice) develop muscle atrophy in both fast and slow muscles, with high expression of muscle-specific E3-ubiquitin ligases, atrogin-1 and MuRF1, and high levels of ROS. Vitamin C supplementation was able to recover the SMP30-knockout muscle atrophy phenotype [157], highlighting its direct involvement in the maintenance of skeletal muscle homeostasis.
- Vitamin D certainly plays an important role in skeletal muscle physiology. Vitamin D deficiency in humans leads to muscle weakness and myalgia that can be reverted by vitamin D replenishment [158]. Vitamin D supplementation is associated with a significant increase in muscle mass and function in older adults with sarcopenia, especially for those with a significant baseline vitamin D deficiency [159,160,161,162]. At the molecular levels, skeletal muscle seems to express the vitamin D receptor, which mediates vitamin D-dependent signaling affecting calcium handling [163]. In addition to beneficial effects on age-related sarcopenia (revised in [164]), vitamin D helps muscle recovery following strenuous muscular activity. However, when vitamin D was supplemented in ALS, negative results prevailed over beneficial findings [165,166,167,168].
- Trolox, the cell-permeable derivative of vitamin E, was not able to exert any protective effects on neurogenic muscle atrophy in mice [62], despite evidence that the combined supplementation of whey protein, vitamin D and E can significantly improve muscle mass, strength, and markers of protein anabolism in sarcopenic subjects [169]. Two studies acknowledged the importance of oxidative stress in neurogenic muscle atrophy: the divergent conclusions come from different treatments (single or combined) and different models (mice vs. humans).
| Functional Nutrient | Disease or Condition | Experimental Model | Mechanism of Action | References |
|---|---|---|---|---|
| Beta-carotene | Nerve rescission | Mice | - Represses the UPS activation | [155] |
| Vitamin C | Sarcopenia | SMP30-KO mice | - Hampers the UPS activation | [157] |
| Vitamin D | Sarcopenia | Elderly human subjects | - Improves muscle mass and strength | [159,160,161,162] |
| Whey protein + vitamin D + vitamin E | Sarcopenia | Elderly human subjects | - Increase muscle mass, muscle strength, and anabolic markers | [169] |
2.4. Plant-Derived Ingredients
- Geranylgeraniol (GGOH), is a plant-derived isoprenoid with some beneficial effects for skeletal muscle mass. GGOH administration reduced the loss of myofiber size upon denervation in rat gastrocnemius muscle by affecting the expression of atrogin-1 [173], without enhancing muscle growth, even though it had previously shown a positive effect on C2C12 myoblast differentiation in vitro [174]. In addition, GGOH may interfere with the NF-κB and/or testosterone signaling in skeletal muscle, as demonstrated in other cell types [174,175], thus contributing to the protection against neurogenic muscle atrophy.
- Capsaicin is a chili pepper-derived extract with analgesic properties. Its protective action against neurogenic muscle atrophy has been described in a study [176]. Capsaicin administration, used as an agonist of the transient receptor potential cation channel, subfamily V, member 1 (TRPV1), induced muscle hypertrophy and alleviated denervation-induced atrophy in both fast and slow murine muscles. Activated TRPV1 increased intracellular Ca2+ concentration leading to mTOR activation and muscle biosynthesis [176].
- Polyphenols are a wide group of plant-derived organic compounds found in fruits, vegetables, coffee, tea, and whole grains. They are believed to be potential therapeutic agents for inhibiting muscle atrophy and improving muscle mass and strength [172]. Polyphenols act primarily as antioxidants and anti-inflammatory agents, thus inhibiting muscle atrophy-related genes and promoting the activation of the IGF-1 signaling pathway [172,177]. In addition, in vitro and in vivo observations proved the neuroprotective effects of these bioactive compounds by improving mitochondrial biogenesis and function, reducing toxic protein aggregates and microglia and astrocytes inflammation, and overall favoring motor neuron survival [178]. Some examples are reported below.
- Tomatidine is the metabolite obtained from α-tomatine, a glycoalkaloid abundantly present in tomato plants. Strikingly, the mRNA expression signature of tomatidine negatively correlates to that one of skeletal muscle atrophy upon fasting and spinal cord injury [210], suggesting that tomatidine might exert an anti-atrophic effect on skeletal muscle. Indeed, tomatine administration induced functional muscle hypertrophy, both in vitro and in vivo, accompanied by reduced adiposity, by activating mTORC1 signaling [210]. By enhancing protein synthesis and mitochondriogenesis, tomatidine also prevented muscle atrophy induced by fasting or immobilization in mice [210]. In C. elegans, tomatidine improves muscle function during aging by activating mitophagy and antioxidant cellular defenses [211]. Based on these promising findings, the use of tomatidine to counteract neurogenic muscle atrophy should be further investigated, along with the delineation of the molecular mechanisms underpinning the atrophy rescue.
- Tinospora cordifolia is a plant found in tropical and sub-tropical parts of Asia, Africa, and Australia, whose extract (TCE) has been widely used in ancient Ayurvedic and Tibetan medicine due to its high antioxidant activity [212]. TCE supplementation prevented neurogenic muscle atrophy by enhancing protein synthesis by antagonizing the proteolytic pathways (calpain and UPS), and by enhancing the oxidative stress response in both slow and fast mouse muscles [213].
| Functional Nutrient | Disease or Condition | Experimental Model | Mechanism of Action | References |
|---|---|---|---|---|
| Geranylgeraniol | Nerve rescission | Rats | - Interferes with the UPS activation | [173] |
| Capsaicin | Nerve rescission | Mice | - Increases [Ca2+]i leading to mTOR activation and muscle biosynthesis | [176] |
| Isoflavones | TNF-α-induced muscle atrophy Nerve rescission | C2C12 myotubes Mice | - Interferes with the activation of MuRF1 - Interferes with the apoptosis-dependent signaling | [179,180] |
| Curcumin | ALS | Mice ALS patients | - Decreases amyloid formation - Diminishes oxidative stress | [187,217] |
| Resveratrol | Nerve rescission | Mice | - Blunts the UPS and autophagy activation | [195] |
| Sciatic nerve crush injury | Rats | - Neuroprotective functions | [196] | |
| AVNs | TNF-α-induced muscle atrophy | C2C12 cells | - Inhibits NF-kB activation, ROS production, and proinflammatory cytokine expression | [199,202] |
| Quercetin | TNF-α-induced muscle atrophy Nerve rescission | C2C12 myotubes Mice | - Inhibits NF-kB activation - Activates HO-1 - Increases mitochondriogenesis and function | [203,204] |
| Epicatechin | Sarcopenia | Aged mice | - Increases protein synthesis - Improves the oxidative stress response - Prevents UPS activation | [205] |
| Epigallocate-chin-3-gallate | Aged rats | - Prevents UPS activation - Reduces myostatin expression - Increases the anabolic pathway | [206] | |
| Apigenin | Nerve rescission | Mice | - Inhibits UPS activation - Reduces inflammation | [207] |
| Genistein | Nerve rescission | Mice | - Prevents UPS activation | [208] |
| 8-prenylnaringenin | Nerve rescission | Mice | - Activates the AKT anabolic pathway - Interferes with UPS activation | [209] |
| Tomatidine | Sarcopenia | C. elegans | - Activates mitophagy - Reduces oxidative stress | [211] |
| Tinospora cordifolia | Nerve rescission | Mice | - Enhances protein synthesis - Antagonizes the proteolytic pathways - Increases the oxidative stress response | [213] |
| Salidroside | Nerve rescission | Rat | - Anti-inflammatory properties | [216] |
2.5. Prebiotics, Probiotics and Dietary Fibers
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Daou, N.; Hassani, M.; Matos, E.; De Castro, G.S.; Costa, R.G.F.; Seelaender, M.; Moresi, V.; Rocchi, M.; Adamo, S.; Li, Z.; et al. Displaced Myonuclei in Cancer Cachexia Suggest Altered Innervation. Int. J. Mol. Sci. 2020, 21, 1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartori, R.; Hagg, A.; Zampieri, S.; Armani, A.; Winbanks, C.E.; Viana, L.R.; Haidar, M.; Watt, K.I.; Qian, H.; Pezzini, C.; et al. Perturbed BMP Signaling and Denervation Promote Muscle Wasting in Cancer Cachexia. Sci. Transl. Med. 2021, 13, eaay9592. [Google Scholar] [CrossRef] [PubMed]
- Ehmsen, J.T.; Höke, A. Cellular and Molecular Features of Neurogenic Skeletal Muscle Atrophy. Exp. Neurol. 2020, 331, 113379. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; Kern, H.; Rossini, K.; Hofer, C.; Mayr, W.; Carraro, U.; Protasi, F. Structural Differentiation of Skeletal Muscle Fibers in the Absence of Innervation in Humans. Proc. Natl. Acad. Sci. USA 2007, 104, 19339–19344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madaro, L.; Passafaro, M.; Sala, D.; Etxaniz, U.; Lugarini, F.; Proietti, D.; Alfonsi, M.V.; Nicoletti, C.; Gatto, S.; De Bardi, M.; et al. Denervation-Activated STAT3–IL-6 Signalling in Fibro-Adipogenic Progenitors Promotes Myofibres Atrophy and Fibrosis. Nat. Cell Biol. 2018, 20, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Dedkov, E.I.; Borisov, A.B.; Carlson, B.M. Dynamics of Postdenervation Atrophy of Young and Old Skeletal Muscles: Differential Responses of Fiber Types and Muscle Types. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Soendenbroe, C.; Andersen, J.L.; Mackey, A.L. Muscle-Nerve Communication and the Molecular Assessment of Human Skeletal Muscle Denervation with Aging. Am. J. Physiol. Cell Physiol. 2021, 321, C317–C329. [Google Scholar] [CrossRef]
- Carraro, U.; Boncompagni, S.; Gobbo, V.; Rossini, K.; Zampieri, S.; Mosole, S.; Ravara, B.; Nori, A.; Stramare, R.; Ambrosio, F.; et al. Persistent Muscle Fiber Regeneration in Long Term Denervation. Past, Present, Future. Eur. J. Transl. Myol. 2015, 25, 77. [Google Scholar] [CrossRef]
- Mosole, S.; Rossini, K.; Kern, H.; Löfler, S.; Fruhmann, H.; Vogelauer, M.; Burggraf, S.; Grim-Stieger, M.; Cvečka, J.; Hamar, D.; et al. Reinnervation of Vastus Lateralis Is Increased Significantly in Seniors (70-Years Old) with a Lifelong History of High-Level Exercise (2013, Revisited Here in 2022). Eur. J. Transl. Myol. 2022, 32, 10420. [Google Scholar] [CrossRef]
- Coletti, C.; Acosta, G.F.; Keslacy, S.; Coletti, D. Exercise-Mediated Reinnervation of Skeletal Muscle in Elderly People: An Update. Eur. J. Transl. Myol. 2022, 32, 10416. [Google Scholar] [CrossRef]
- Feng, F.; Shan, L.; Deng, J.X.; Luo, L.L.; Huang, Q.S. Role of the Notch Signaling Pathway in Fibrosis of Denervated Skeletal Muscle. Curr. Med. Sci. 2019, 39, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Bohnert, K.R.; Tomaz da Silva, M.; Wen, Y.; Bhat, R.; Roy, A.; Kumar, A. MyD88-Mediated Signaling Intercedes in Neurogenic Muscle Atrophy through Multiple Mechanisms. FASEB J. 2021, 35, e21821. [Google Scholar] [CrossRef] [PubMed]
- Argadine, H.M.; Mantilla, C.B.; Zhan, W.Z.; Sieck, G.C. Intracellular Signaling Pathways Regulating Net Protein Balance Following Diaphragm Muscle Denervation. Am. J. Physiol. Cell Physiol. 2011, 300, 318–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siu, P.M.; Alway, S.E. Response and Adaptation of Skeletal Muscle to Denervation Stress: The Role of Apoptosis in Muscle Loss. Front. Biosci. Landmark Ed. 2009, 14, 432–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.M.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Erqian, N.; Dharmarajan, K.; et al. Identification of Ubiquitin Ligases Required for Skeletal Muscle Atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Bachiller, S.; Alonso-bellido, I.M.; Real, L.M.; Pérez-villegas, E.M.; Venero, J.L.; Deierborg, T.; Armengol, J.Á.; Ruiz, R. The Ubiquitin Proteasome System in Neuromuscular Disorders: Moving Beyond Movement. Int. J. Mol. Sci. 2020, 21, 6429. [Google Scholar] [CrossRef]
- Damgaard, R.B. The Ubiquitin System: From Cell Signalling to Disease Biology and New Therapeutic Opportunities. Cell Death Differ. 2021, 28, 423–426. [Google Scholar] [CrossRef]
- Sartori, R.; Schirwis, E.; Blaauw, B.; Bortolanza, S.; Zhao, J.; Enzo, E.; Stantzou, A.; Mouisel, E.; Toniolo, L.; Ferry, A.; et al. BMP Signaling Controls Muscle Mass. Nat. Genet. 2013, 45, 1309–1318. [Google Scholar] [CrossRef]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of Autophagy and the Ubiquitin–Proteasome System by the FoxO Transcriptional Network during Muscle Atrophy. Nat. Commun. 2015, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.; Brault, J.J.; Gygi, S.P.; Glass, D.J.; Valenzuela, D.M.; Gartner, C.; Latres, E.; Goldberg, A.L. During Muscle Atrophy, Thick, but Not Thin, Filament Components Are Degraded by MuRF1-Dependent Ubiquitylation. J. Cell Biol. 2009, 185, 1083–1095. [Google Scholar] [CrossRef]
- Yuasa, K.; Okubo, K.; Yoda, M.; Otsu, K.; Ishii, Y.; Nakamura, M.; Itoh, Y.; Horiuchi, K. Targeted Ablation of P38α MAPK Suppresses Denervation-Induced Muscle Atrophy. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Inoki, K.; Lee, M.; Wright, E.; Khuong, A.; Khuong, A.; Sugiarto, S.; Garner, M.; Paik, J.; DePinho, R.A.; et al. MTORC1 Promotes Denervation-Induced Muscle Atrophy through a Mechanism Involving the Activation of FoxO and E3 Ubiquitin Ligases. Sci. Signal. 2014, 7, ra18. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo Transcription Factors Induce the Atrophy-Related Ubiquitin Ligase Atrogin-1 and Cause Skeletal Muscle Atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Odeh, M.; Tamir-Livne, Y.; Haas, T.; Bengal, E. P38α MAPK Coordinates the Activities of Several Metabolic Pathways That Together Induce Atrophy of Denervated Muscles. FEBS J. 2020, 287, 73–93. [Google Scholar] [CrossRef]
- Vainshtein, A.; Sandri, M. Signaling Pathways That Control Muscle Mass. Int. J. Mol. Sci. 2020, 21, 4759. [Google Scholar] [CrossRef]
- Moresi, V.; Adamo, S.; Berghella, L. The JAK/STAT Pathway in Skeletal Muscle Pathophysiology. Front. Physiol. 2019, 10, 500. [Google Scholar] [CrossRef] [Green Version]
- Moresi, V.; Williams, A.H.; Meadows, E.; Flynn, J.M.; Potthoff, M.J.; McAnally, J.; Shelton, J.M.; Backs, J.; Klein, W.H.; Richardson, J.A.; et al. Myogenin and Class II HDACs Control Neurogenic Muscle Atrophy by Inducing E3 Ubiquitin Ligases. Cell 2010, 143, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Shi, X.; Lu, H.; Xia, B.; Li, Y.; Li, X.; Zhang, Q.; Yang, G. RNA-Seq Transcriptome Analysis of Extensor Digitorum Longus and Soleus Muscles in Large White Pigs. Mol. Genet. Genom. 2015, 291, 687–701. [Google Scholar] [CrossRef]
- Papinski, D.; Kraft, C. Regulation of Autophagy By Signaling Through the Atg1/ULK1 Complex. J. Mol. Biol. 2016, 428, 1725–1741. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef]
- McGrath, M.J.; Eramo, M.J.; Gurung, R.; Sriratana, A.; Gehrig, S.M.; Lynch, G.S.; Lourdes, S.R.; Koentgen, F.; Feeney, S.J.; Lazarou, M.; et al. Defective Lysosome Reformation during Autophagy Causes Skeletal Muscle Disease. J. Clin. Investig. 2021, 131, e135124. [Google Scholar] [CrossRef] [PubMed]
- González-Ramos, M.; Calleros, L.; López-Ongil, S.; Raoch, V.; Griera, M.; Rodríguez-Puyol, M.; De Frutos, S.; Rodríguez-Puyol, D. HSP70 Increases Extracellular Matrix Production by Human Vascular Smooth Muscle through TGF-Β1 up-Regulation. Int. J. Biochem. Cell Biol. 2013, 45, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Castets, P.; Lin, S.; Rion, N.; Di Fulvio, S.; Romanino, K.; Guridi, M.; Frank, S.; Tintignac, L.A.; Sinnreich, M.; Rüegg, M.A. Sustained Activation of MTORC1 in Skeletal Muscle Inhibits Constitutive and Starvation-Induced Autophagy and Causes a Severe, Late-Onset Myopathy. Cell Metab. 2013, 17, 731–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnio, S.; LoVerso, F.; Baraibar, M.A.; Longa, E.; Khan, M.M.; Maffei, M.; Reischl, M.; Canepari, M.; Loefler, S.; Kern, H.; et al. Autophagy Impairment in Muscle Induces Neuromuscular Junction Degeneration and Precocious Aging. Cell Rep. 2014, 8, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Moresi, V.; Carrer, M.; Grueter, C.E.; Rifki, O.F.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Histone Deacetylases 1 and 2 Regulate Autophagy Flux and Skeletal Muscle Homeostasis in Mice. Proc. Natl. Acad. Sci. USA 2012, 109, 1649–1654. [Google Scholar] [CrossRef] [Green Version]
- Pigna, E.; Berardi, E.; Aulino, P.; Rizzuto, E.; Zampieri, S.; Carraro, U.; Kern, H.; Merigliano, S.; Gruppo, M.; Mericskay, M.; et al. Aerobic Exercise and Pharmacological Treatments Counteract Cachexia by Modulating Autophagy in Colon Cancer. Sci. Rep. 2016, 6, 26991. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, H.; Zhang, D.; Luo, W.; Liu, R.; Xu, D.; Diao, L.; Liao, L.; Liu, Z. Phosphorylation of ULK1 Affects Autophagosome Fusion and Links Chaperone-Mediated Autophagy to Macroautophagy. Nat. Commun. 2018, 9, 3492. [Google Scholar] [CrossRef]
- Nichenko, A.S.; Sorensen, J.R.; Southern, W.M.; Qualls, A.E.; Schifino, A.G.; McFaline-Figueroa, J.; Blum, J.E.; Tehrani, K.F.; Yin, H.; Mortensen, L.J.; et al. Lifelong Ulk1-Mediated Autophagy Deficiency in Muscle Induces Mitochondrial Dysfunction and Contractile Weakness. Int. J. Mol. Sci. 2021, 22, 1937. [Google Scholar] [CrossRef]
- Kamada, Y.; Yoshino, K.; Kondo, C.; Kawamata, T.; Oshiro, N.; Yonezawa, K.; Ohsumi, Y. Tor Directly Controls the Atg1 Kinase Complex to Regulate Autophagy. Mol. Cell. Biol. 2010, 30, 1049–1058. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, A.M.J.; Csibi, A.; Raibon, A.; Cornille, K.; Gay, S.; Bernardi, H.; Candau, R. AMPK Promotes Skeletal Muscle Autophagy through Activation of Forkhead FoxO3a and Interaction with Ulk1. J. Cell. Biochem. 2012, 113, 695–710. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Takeda, K.; Tamura, Y.; Fujimaki, S.; Takemasa, T.; Hatta, H. Nrf2 Deficiency Does Not Affect Denervation-Induced Alterations in Mitochondrial Fission and Fusion Proteins in Skeletal Muscle. Physiol. Rep. 2016, 4, e13064. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Brault, J.J.; Schild, A.; Cao, P.; Sandri, M.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. FoxO3 Coordinately Activates Protein Degradation by the Autophagic/Lysosomal and Proteasomal Pathways in Atrophying Muscle Cells. Cell Metab. 2007, 6, 472–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quy, P.N.; Kuma, A.; Pierres, P.; Mizushima, N. Proteasome-Dependent Activation of Mammalian Target of Rapamycin Complex 1 (MTORC1) Is Essential for Autophagy Suppression and Muscle Remodeling Following Denervation. J. Biol. Chem. 2013, 288, 1125–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S.; Zhai, B.; Gygi, S.P.; Goldberg, A.L. Ubiquitylation by Trim32 Causes Coupled Loss of Desmin, Z-Bands, and Thin Filaments in Muscle Atrophy. J. Cell Biol. 2012, 198, 575–589. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Antonioli, M.; Fusco, C.; Liu, Y.; Mari, M.; Orhon, I.; Refolo, G.; Germani, F.; Corazzari, M.; Romagnoli, A.; et al. Autophagy Induction in Atrophic Muscle Cells Requires ULK1 Activation by TRIM32 through Unanchored K63-Linked Polyubiquitin Chains. Sci. Adv. 2019, 5, eaau8857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masiero, E.; Agatea, L.; Mammucari, C.; Blaauw, B.; Loro, E.; Komatsu, M.; Metzger, D.; Reggiani, C.; Schiaffino, S.; Sandri, M. Autophagy Is Required to Maintain Muscle Mass. Cell Metab. 2009, 10, 507–515. [Google Scholar] [CrossRef]
- Pigna, E.; Sanna, K.; Coletti, D.; Li, Z.; Parlakian, A.; Adamo, S.; Moresi, V. Increasing Autophagy Does Not Affect Neurogenic Muscle Atrophy. Eur. J. Transl. Myol. 2018, 28, 248–256. [Google Scholar] [CrossRef]
- Yang, X.; Xue, P.; Liu, X.; Xu, X.; Chen, Z. HMGB1/Autophagy Pathway Mediates the Atrophic Effect of TGF-Β1 in Denervated Skeletal Muscle. Cell Commun. Signal. 2018, 16, 97. [Google Scholar] [CrossRef] [Green Version]
- Kumamoto, T.; Kleese, W.C.; Cong, J.; Goll, D.E.; Pierce, P.R.; Allen, R.E. Localization of the Ca2+-Dependent Proteinases and Their Inhibitor in Normal, Fasted, and Denervated Rat Skeletal Muscle. Anat. Rec. 1992, 232, 60–77. [Google Scholar] [CrossRef]
- Aweida, D.; Rudesky, I.; Volodin, A.; Shimko, E.; Cohen, S. GSK3-β Promotes Calpain-1–Mediated Desmin Filament Depolymerization and Myofibril Loss in Atrophy. J. Cell Biol. 2018, 217, 3698–3714. [Google Scholar] [CrossRef]
- Chen, F.; Qian, L.; Yang, Z.H.; Huang, Y.; Ngo, S.T.; Ruan, N.J.; Wang, J.; Schneider, C.; Noakes, P.G.; Ding, Y.Q.; et al. Rapsyn Interaction with Calpain Stabilizes AChR Clusters at the Neuromuscular Junction. Neuron 2007, 55, 247–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groshong, J.S.; Spencer, M.J.; Bhattacharyya, B.J.; Kudryashova, E.; Vohra, B.P.S.; Zayas, R.; Wollmann, R.L.; Miller, R.J.; Gomez, C.M. Calpain Activation Impairs Neuromuscular Transmission in a Mouse Model of the Slow-Channel Myasthenic Syndrome. J. Clin. Investig. 2007, 117, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.; Silveira, W.A.; Gonçalves, D.A.; Schavinski, A.Z.; Khan, M.M.; Zanon, N.M.; Diaz, M.B.; Rudolf, R.; Kettelhut, I.C.; Navegantes, L.C. A−Calcitonin Gene-Related Peptide Inhibits Autophagy and Calpain Systems and Maintains the Stability of Neuromuscular Junction in Denervated Muscles. Mol. Metab. 2019, 28, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Dargelos, E.; Brulé, C.; Combaret, L.; Hadj-Sassi, A.; Dulong, S.; Poussard, S.; Cottin, P. Involvement of the Calcium-Dependent Proteolytic System in Skeletal Muscle Aging. Exp. Gerontol. 2007, 42, 1088–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enns, D.L.; Raastad, T.; Ugelstad, I.; Belcastro, A.N. Calpain/Calpastatin Activities and Substrate Depletion Patterns during Hindlimb Unweighting and Reweighting in Skeletal Muscle. Eur. J. Appl. Physiol. 2007, 100, 445–455. [Google Scholar] [CrossRef]
- Samengo, G.; Avik, A.; Fedor, B.; Whittaker, D.; Myung, K.H.; Wehling-Henricks, M.; Tidball, J.G. Age-Related Loss of Nitric Oxide Synthase in Skeletal Muscle Causes Reductions in Calpain S-Nitrosylation That Increase Myofibril Degradation and Sarcopenia. Aging Cell 2012, 11, 1036–1045. [Google Scholar] [CrossRef] [Green Version]
- Schroder, E.A.; Wang, L.; Wen, Y.; Callahan, L.A.P.; Supinski, G.S. Skeletal Muscle-Specific Calpastatin Overexpression Mitigates Muscle Weakness in Aging and Extends Life Span. J. Appl. Physiol. 2021, 131, 630–642. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive Oxygen Species Are Essential for Autophagy and Specifically Regulate the Activity of Atg4. EMBO J. 2007, 26, 1749–1760. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Nelson, W.B.; Hudson, M.B. Exercise-Induced Oxidative Stress in Humans: Cause and Consequences. Free Radic. Biol. Med. 2011, 51, 942–950. [Google Scholar] [CrossRef]
- Ardite, E.; Barbera, J.A.; Roca, J.; Fernández-Checa, J.C. Glutathione Depletion Impairs Myogenic Differentiation of Murine Skeletal Muscle C2C12 Cells through Sustained NF-ΚB Activation. Am. J. Pathol. 2004, 165, 719–728. [Google Scholar] [CrossRef]
- O’Leary, M.F.N.; Hood, D.A. Effect of Prior Chronic Contractile Activity on Mitochondrial Function and Apoptotic Protein Expression in Denervated Muscle. J. Appl. Physiol. 2008, 105, 114–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pigna, E.; Greco, E.; Morozzi, G.; Grottelli, S.; Rotini, A.; Minelli, A.; Fulle, S.; Adamo, S.; Mancinelli, R.; Bellezza, I.; et al. Denervation Does Not Induce Muscle Atrophy Through Oxidative Stress. Eur. J. Transl. Myol. 2017, 27, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, M.F.N.; Hood, D.A. Denervation-Induced Oxidative Stress and Autophagy Signaling in Muscle. Autophagy 2009, 5, 230–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic Lateral Sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef] [Green Version]
- Halter, B.; Gonzalez de Aguilar, J.L.; Rene, F.; Petri, S.; Fricker, B.; Echaniz-Laguna, A.; Dupuis, L.; Larmet, Y.; Loeffler, J.P. Oxidative Stress in Skeletal Muscle Stimulates Early Expression of Rad in a Mouse Model of Amyotrophic Lateral Sclerosis. Free Radic. Biol. Med. 2010, 48, 915–923. [Google Scholar] [CrossRef]
- Dobrowolny, G.; Aucello, M.; Rizzuto, E.; Beccafico, S.; Mammucari, C.; Boncompagni, S.; Belia, S.; Wannenes, F.; Nicoletti, C.; Del Prete, Z.; et al. Skeletal Muscle Is a Primary Target of SOD1G93A-Mediated Toxicity. Cell Metab. 2008, 8, 425–436. [Google Scholar] [CrossRef]
- Dobrowolny, G.; Martini, M.; Scicchitano, B.M.; Romanello, V.; Boncompagni, S.; Nicoletti, C.; Pietrangelo, L.; De Panfilis, S.; Catizone, A.; Bouchè, M.; et al. Muscle Expression of SOD1 G93A Triggers the Dismantlement of Neuromuscular Junction via PKC-Theta. Antioxid. Redox Signal. 2018, 28, 1105–1119. [Google Scholar] [CrossRef]
- Orrell, R.W.; Lane, R.J.M.; Ross, M. A Systematic Review of Antioxidant Treatment for Amyotrophic Lateral Sclerosis/Motor Neuron Disease. Amyotroph. Lateral Scler. 2008, 9, 195–211. [Google Scholar] [CrossRef]
- Yoon, M.S. MTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef] [Green Version]
- Goodman, C.A. Role of MTORC1 in Mechanically Induced Increases in Translation and Skeletal Muscle Mass. J. Appl. Physiol. 2019, 127, 581–590. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. MTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [Green Version]
- Hara, K.; Maruki, Y.; Long, X.; Yoshino, K.I.; Oshiro, N.; Hidayat, S.; Tokunaga, C.; Avruch, J.; Yonezawa, K. Raptor, a Binding Partner of Target of Rapamycin (TOR), Mediates TOR Action. Cell 2002, 110, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Sarbassov, D.D.; Ali, S.M.; King, J.E.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. MTOR Interacts with Raptor to Form a Nutrient-Sensitive Complex That Signals to the Cell Growth Machinery. Cell 2002, 110, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Mieulet, V.; Roceri, M.; Espeillac, C.; Sotiropoulos, A.; Ohanna, M.; Oorschot, V.; Klumperman, J.; Sandri, M.; Pende, M. S6 Kinase Inactivation Impairs Growth and Translational Target Phosphorylation in Muscle Cells Maintaining Proper Regulation of Protein Turnover. Am. J. Physiol. Cell Physiol. 2007, 293, C712–C722. [Google Scholar] [CrossRef] [Green Version]
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentzinger, C.F.; Romanino, K.; Cloëtta, D.; Lin, S.; Mascarenhas, J.B.; Oliveri, F.; Xia, J.; Casanova, E.; Costa, C.F.; Brink, M.; et al. Skeletal Muscle-Specific Ablation of Raptor, but Not of Rictor, Causes Metabolic Changes and Results in Muscle Dystrophy. Cell Metab. 2008, 8, 411–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risson, V.; Mazelin, L.; Roceri, M.; Sanchez, H.; Moncollin, V.; Corneloup, C.; Richard-Bulteau, H.; Vignaud, A.; Baas, D.; Defour, A.; et al. Muscle Inactivation of MTOR Causes Metabolic and Dystrophin Defects Leading to Severe Myopathy. J. Cell Biol. 2009, 187, 859. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, V.; Alliouachene, S.; Sotiropoulos, A.; Sobering, A.; Athea, Y.; Djouadi, F.; Miraux, S.; Thiaudière, E.; Foretz, M.; Viollet, B.; et al. S6 Kinase Deletion Suppresses Muscle Growth Adaptations to Nutrient Availability by Activating AMP Kinase. Cell Metab. 2007, 5, 476–487. [Google Scholar] [CrossRef] [Green Version]
- Kleinert, M.; Parker, B.L.; Chaudhuri, R.; Fazakerley, D.J.; Serup, A.; Thomas, K.C.; Krycer, J.R.; Sylow, L.; Fritzen, A.M.; Hoffman, N.J.; et al. MTORC2 and AMPK Differentially Regulate Muscle Triglyceride Content via Perilipin 3. Mol. Metab. 2016, 5, 646. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, E.M.; Andres-Mateos, E.; Mejias, R.; Simmers, J.L.; Mi, R.; Park, J.S.; Ying, S.; Hoke, A.; Lee, S.J.; Cohn, R.D. Denervation Atrophy Is Independent from Akt and MTOR Activation and Is Not Rescued by Myostatin Inhibition. Dis. Model. Mech. 2014, 7, 471–481. [Google Scholar] [CrossRef]
- You, J.S.; Kim, K.; Steinert, N.D.; Chen, J.; Hornberger, T.A. MTORC1 Mediates Fiber Type-Specific Regulation of Protein Synthesis and Muscle Size during Denervation. Cell Death Discov. 2021, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/MTOR Pathway Is a Crucial Regulator of Skeletal Muscle Hypertrophy and Can Prevent Muscle Atrophy in Vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.H.; Rando, T.A. Induction of Autophagy Supports the Bioenergetic Demands of Quiescent Muscle Stem Cell Activation. EMBO J. 2014, 33, 2782–2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argadine, H.M.; Hellyer, N.J.; Mantilla, C.B.; Zhan, W.Z.; Sieck, G.C. The Effect of Denervation on Protein Synthesis and Degradation in Adult Rat Diaphragm Muscle. J. Appl. Physiol. 2009, 107, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Scientific Concepts of Functional Foods in Europe. Consensus Document-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/10999022/ (accessed on 29 July 2022).
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The Role of Omega-3 in the Prevention and Treatment of Sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef] [Green Version]
- Tieland, M.; Franssen, R.; Dullemeijer, C.; van Dronkelaar, C.; Kim, H.K.; Ispoglou, T.; Zhu, K.; Prince, R.L.; van Loon, L.J.C.; de Groot, L.C.P.G.M. The Impact of Dietary Protein or Amino Acid Supplementation on Muscle Mass and Strength in Elderly People: Individual Participant Data and Meta-Analysis of RCT’s. J. Nutr. Health Aging 2017, 21, 994–1001. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Milan, A.M.; Mitchell, S.M.; Zeng, N.; Ramzan, F.; Sharma, P.; Knowles, S.O.; Roy, N.C.; Sjödin, A.; Wagner, K.H.; et al. The Effects of Dietary Protein Intake on Appendicular Lean Mass and Muscle Function in Elderly Men: A 10-Wk Randomized Controlled Trial. Am. J. Clin. Nutr. 2017, 106, 1375–1383. [Google Scholar] [CrossRef] [Green Version]
- Cawood, A.L.; Elia, M.; Stratton, R.J. Systematic Review and Meta-Analysis of the Effects of High Protein Oral Nutritional Supplements. Ageing Res. Rev. 2012, 11, 278–296. [Google Scholar] [CrossRef]
- Tieland, M.; van de Rest, O.; Dirks, M.L.; van der Zwaluw, N.; Mensink, M.; van Loon, L.J.C.; de Groot, L.C.P.G.M. Protein Supplementation Improves Physical Performance in Frail Elderly People: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Med. Dir. Assoc. 2012, 13, 720–726. [Google Scholar] [CrossRef]
- Zhu, K.; Kerr, D.A.; Meng, X.; Devine, A.; Solah, V.; Binns, C.W.; Prince, R.L. Two-Year Whey Protein Supplementation Did Not Enhance Muscle Mass and Physical Function in Well-Nourished Healthy Older Postmenopausal Women. J. Nutr. 2015, 145, 2520–2526. [Google Scholar] [CrossRef]
- Kim, B.; Jin, Y.; Kim, S.H.; Park, Y. Association between Macronutrient Intake and Amyotrophic Lateral Sclerosis Prognosis. Nutr. Neurosci. 2018, 23, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Genton, L.; Viatte, V.; Janssens, J.P.; Héritier, A.C.; Pichard, C. Nutritional State, Energy Intakes and Energy Expenditure of Amyotrophic Lateral Sclerosis (ALS) Patients. Clin. Nutr. 2011, 30, 553–559. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-Chain Amino Acid Supplementation Promotes Survival and Supports Cardiac and Skeletal Muscle Mitochondrial Biogenesis in Middle-Aged Mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, G.H.; Tsai, M.C.; Tsai, H.W.; Chang, C.C.; Hou, W.H. Effects of Branched-Chain Amino Acid-Rich Supplementation on EWGSOP2 Criteria for Sarcopenia in Older Adults: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2022, 61, 637–651. [Google Scholar] [CrossRef]
- Buondonno, I.; Sassi, F.; Carignano, G.; Dutto, F.; Ferreri, C.; Pili, F.G.; Massaia, M.; Nisoli, E.; Ruocco, C.; Porrino, P.; et al. From Mitochondria to Healthy Aging: The Role of Branched-Chain Amino Acids Treatment: MATeR a Randomized Study. Clin. Nutr. 2020, 39, 2080–2091. [Google Scholar] [CrossRef] [Green Version]
- Tandan, R.; Bromberg, M.B.; Forshew, D.; Fries, T.J.; Badger, G.J.; Carpenter, J.; Krusinski, P.B.; Betts, E.F.; Arciero, K.; Nau, K. A Controlled Trial of Amino Acid Therapy in Amyotrophic Lateral Sclerosis: I. Clinical, Functional, and Maximum Isometric Torque Data. Neurology 1996, 47, 1220–1226. [Google Scholar] [CrossRef]
- Rondanelli, M.; Nichetti, M.; Peroni, G.; Faliva, M.A.; Naso, M.; Gasparri, C.; Perna, S.; Oberto, L.; Di Paolo, E.; Riva, A.; et al. Where to Find Leucine in Food and How to Feed Elderly With Sarcopenia in Order to Counteract Loss of Muscle Mass: Practical Advice. Front. Nutr. 2021, 7, 383. [Google Scholar] [CrossRef]
- Ribeiro, C.B.; Christofoletti, D.C.; Pezolato, V.A.; de Cássia Marqueti Durigan, R.; Prestes, J.; Tibana, R.A.; Pereira, E.C.L.; de Sousa Neto, I.V.; Durigan, J.L.Q.; da Silva, C.A. Leucine Minimizes Denervation-Induced Skeletal Muscle Atrophy of Rats through Akt/Mtor Signaling Pathways. Front. Physiol. 2015, 6, 73. [Google Scholar] [CrossRef] [Green Version]
- Martínez-arnau, F.M.; Fonfría-vivas, R.; Cauli, O. Beneficial Effects of Leucine Supplementation on Criteria for Sarcopenia: A Systematic Review. Nutrition 2019, 11, 2504. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Buigues, C.; Castillo, Y.; Molina, P.; Hoogland, A.J.; van Doesburg, F.; Pruimboom, L.; Fernández-Garrido, J.; Cauli, O. Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-Controlled Clinical Trial. Nutrition 2020, 12, 932. [Google Scholar] [CrossRef]
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Essential Amino Acids Increase MicroRNA-499, -208b, and -23a and Downregulate Myostatin and Myocyte Enhancer Factor 2C MRNA Expression in Human Skeletal Muscle. J. Nutr. 2009, 139, 2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, R.J.; Cagnin, S.; Chemello, F.; Silvestrin, M.; Musaro, A.; De Pitta, C.; Lanfranchi, G.; Sandri, M. Involvement of MicroRNAs in the Regulation of Muscle Wasting during Catabolic Conditions. J. Biol. Chem. 2014, 289, 21909–21925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanchi, N.E.; Gerlinger-Romero, F.; Guimarães-Ferreira, L.; De Siqueira Filho, M.A.; Felitti, V.; Lira, F.S.; Seelaender, M.; Lancha, A.H. HMB Supplementation: Clinical and Athletic Performance-Related Effects and Mechanisms of Action. Amino Acids 2011, 40, 1015–1025. [Google Scholar] [CrossRef]
- Baier, S.; Johannsen, D.; Abumrad, N.; Rathmacher, J.A.; Nissen, S.; Flakoll, P. Year-Long Changes in Protein Metabolism in Elderly Men and Women Supplemented with a Nutrition Cocktail of Beta-Hydroxy-Beta-Methylbutyrate (HMB), L-Arginine, and L-Lysine. JPEN. J. Parenter. Enteral Nutr. 2009, 33, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Wilund, K.; Fitschen, P.J.; Jeejeebhoy, K.; Agarwala, R.; Drover, J.W.; Mourtzakis, M. Elderly Persons with ICU-Acquired Weakness: The Potential Role for β-Hydroxy-β-Methylbutyrate (HMB) Supplementation? J. Parenter. Enter. Nutr. 2014, 38, 567–575. [Google Scholar] [CrossRef]
- De Angelus, N.; Riela, C.; Moeme, M.; Guimarães, A.; Oliveira De Almeida, D.; Maria Queiroz Araujo, E.; Maria, E.; Araujo, Q. Effects of Beta-Hydroxy-Beta-Methylbutyrate Supplementation on Elderly Body Composition and Muscle Strength: A Review of Clinical Trials of Nutrition Course and GENUT, Professor at Post-Graduation Program Interactive Process of Organs and Systems, Salvador, Brazil. Rev. Artic. Ann. Nutr. Metab. 2008, 77, 16–22. [Google Scholar] [CrossRef]
- Phillips, S.M.; Lau, K.J.; D’Souza, A.C.; Nunes, E.A. An Umbrella Review of Systematic Reviews of β-Hydroxy-β-Methyl Butyrate Supplementation in Ageing and Clinical Practice. J. Cachexia. Sarcopenia Muscle 2022, 13, 2265–2275. [Google Scholar] [CrossRef]
- Lin, Z.; Zhao, A.; He, J. Effect of β-Hydroxy-β-Methylbutyrate (HMB) on the Muscle Strength in the Elderly Population: A Meta-Analysis. Front. Nutr. 2022, 9, 914866. [Google Scholar] [CrossRef]
- Candow, D.G.; Chilibeck, P.D.; Forbes, S.C. Creatine Supplementation and Aging Musculoskeletal Health. Endocrine 2014, 45, 354–361. [Google Scholar] [CrossRef]
- Chilibeck, P.; Kaviani, M.; Candow, D.; Zello, G.A. Effect of Creatine Supplementation during Resistance Training on Lean Tissue Mass and Muscular Strength in Older Adults: A Meta-Analysis. Open access J. Sport. Med. 2017, 8, 213–226. [Google Scholar] [CrossRef]
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R.B. Effectiveness of Creatine Supplementation on Aging Muscle and Bone: Focus on Falls Prevention and Inflammation. J. Clin. Med. 2019, 8, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, A.C.; Rosenfeld, J. The Role of Creatine in the Management of Amyotrophic Lateral Sclerosis and Other Neurodegenerative Disorders. CNS Drugs 2004, 18, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Adhihetty, P.J.; Beal, M.F. Creatine and Its Potential Therapeutic Value for Targeting Cellular Energy Impairment in Neurodegenerative Diseases. Neuromolecular Med. 2008, 10, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Ringseis, R.; Keller, J.; Eder, K. Mechanisms Underlying the Anti-Wasting Effect of l-Carnitine Supplementation under Pathologic Conditions: Evidence from Experimental and Clinical Studies. Eur. J. Nutr. 2013, 52, 1421–1442. [Google Scholar] [CrossRef] [PubMed]
- Czyzewski, K.; Stern, L.Z.; Sadeh, M.; Bahl, J.J. Altered Rat Skeletal Muscle Carnitine with Age and after Denervation. Muscle Nerve 1985, 8, 34–37. [Google Scholar] [CrossRef]
- Pesce, V.; Fracasso, F.; Cassano, P.; Lezza, A.M.S.; Cantatore, P.; Gadaleta, M.N. Acetyl-L-Carnitine Supplementation to Old Rats Partially Reverts the Age-Related Mitochondrial Decay of Soleus Muscle by Activating Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1alpha-Dependent Mitochondrial Biogenesis. Rejuvenation Res. 2010, 13, 148–151. [Google Scholar] [CrossRef]
- Iossa, S.; Pina Mollica, M.; Lionetti, L.; Crescenzo, R.; Botta, M.; Barletta, A.; Liverini, G. Acetyl-L-Carnitine Supplementation Differently Influences Nutrient Partitioning, Serum Leptin Concentration and Skeletal Muscle Mitochondrial Respiration in Young and Old Rats. J. Nutr. 2002, 132, 636–642. [Google Scholar] [CrossRef] [Green Version]
- Bernard, A.; Rigault, C.; Mazue, F.; Le Borgne, F.; Demarquoy, J. L-Carnitine Supplementation and Physical Exercise Restore Age-Associated Decline in Some Mitochondrial Functions in the Rat. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1027–1033. [Google Scholar] [CrossRef] [Green Version]
- Kira, Y.; Nishikawa, M.; Ochi, A.; Sato, E.; Inoue, M. L-Carnitine Suppresses the Onset of Neuromuscular Degeneration and Increases the Life Span of Mice with Familial Amyotrophic Lateral Sclerosis. Brain Res. 2006, 1070, 206–214. [Google Scholar] [CrossRef]
- Beghi, E.; Pupillo, E.; Bonito, V.; Buzzi, P.; Caponnetto, C.; Chiò, A.; Corbo, M.; Giannini, F.; Inghilleri, M.; Bella, V.L.; et al. Randomized Double-Blind Placebo-Controlled Trial of Acetyl-L-Carnitine for ALS. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 397–405. [Google Scholar] [CrossRef]
- Perim, P.; Marticorena, F.M.; Ribeiro, F.; Barreto, G.; Gobbi, N.; Kerksick, C.; Dolan, E.; Saunders, B. Can the Skeletal Muscle Carnosine Response to Beta-Alanine Supplementation Be Optimized? Front. Nutr. 2019, 6, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hipkiss, A.R.; Brownson, C. A Possible New Role for the Anti-Ageing Peptide Carnosine. Cell. Mol. Life Sci. CMLS 2000, 57, 747–753. [Google Scholar] [CrossRef]
- Stuerenburg, H.J. The Roles of Carnosine in Aging of Skeletal Muscle and in Neuromuscular Diseases. Biochemistry 2000, 65, 862–865. [Google Scholar]
- Solana-Manrique, C.; Sanz, F.J.; Martínez-Carrión, G.; Paricio, N. Antioxidant and Neuroprotective Effects of Carnosine: Therapeutic Implications in Neurodegenerative Diseases. Antioxidants 2022, 11, 848. [Google Scholar] [CrossRef] [PubMed]
- Budzeń, S.; Szcześniak, D.; Kopeć, W.; Rymaszewska, J. Anserine and Carnosine Supplementation in the Elderly: Effects on Cognitive Functioning and Physical Capacity. Arch. Gerontol. Geriatr. 2014, 59, 485–490. [Google Scholar] [CrossRef]
- Hisatsune, T.; Kaneko, J.; Kurashige, H.; Cao, Y.; Satsu, H.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H. Effect of Anserine/Carnosine Supplementation on Verbal Episodic Memory in Elderly People. J. Alzheimers. Dis. 2016, 50, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Kong, J.; Underwood, C.; Petocz, P.; Hirani, V.; Dawson, B.; O’Leary, F. Systematic Review and Meta-Analysis of the Effect of Protein and Amino Acid Supplements in Older Adults with Acute or Chronic Conditions. Br. J. Nutr. 2018, 119, 527–542. [Google Scholar] [CrossRef]
- Montiel-rojas, D.; Santoro, A.; Nilsson, A.; Franceschi, C.; Capri, M.; Bazzocchi, A.; Battista, G.; de Groot, L.C.P.G.M.; Feskens, E.J.M.; Berendsen, A.A.M.; et al. Beneficial Role of Replacing Dietary Saturated Fatty Acids with Polyunsaturated Fatty Acids in the Prevention of Sarcopenia: Findings from the NU-AGE Cohort. Nutrition 2020, 12, 3079. [Google Scholar] [CrossRef]
- Gray, S.R.; Mittendorfer, B. Fish Oil-Derived n-3 Polyunsaturated Fatty Acids for the Prevention and Treatment of Sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, K.C.; O’Reilly, É.J.; Falcone, G.J.; McCullough, M.L.; Park, Y.; Kolonel, L.N.; Ascherio, A. Dietary ω-3 Polyunsaturated Fatty Acid Intake and Risk for Amyotrophic Lateral Sclerosis. JAMA Neurol. 2014, 71, 1102–1110. [Google Scholar] [CrossRef]
- Veldink, J.H.; Kalmijn, S.; Groeneveld, G.J.; Wunderink, W.; Koster, A.; De Vries, J.H.M.; Van Der Luyt, J.; Wokke, J.H.J.; Van Den Berg, L.H. Intake of Polyunsaturated Fatty Acids and Vitamin E Reduces the Risk of Developing Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2007, 78, 367–371. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary Omega-3 Fatty Acid Supplementation Increases the Rate of Muscle Protein Synthesis in Older Adults: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish Oil-Derived n-3 PUFA Therapy Increases Muscle Mass and Function in Healthy Older Adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipina, C.; Hundal, H.S. Lipid Modulation of Skeletal Muscle Mass and Function. J. Cachexia. Sarcopenia Muscle 2017, 8, 190–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelan, J.; Fritsche, K. Linoleic Acid. Adv. Nutr. 2013, 4, 311–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carotenuto, F.; Coletti, D.; Di Nardo, P.; Teodori, L. α-Linolenic Acid Reduces TNF-Induced Apoptosis in C2C12 Myoblasts by Regulating Expression of Apoptotic Proteins. Eur. J. Transl. Myol. 2016, 26, 6033. [Google Scholar] [CrossRef] [Green Version]
- Carotenuto, F.; Costa, A.; Albertini, M.C.; Rocchi, M.B.L.; Rudov, A.; Coletti, D.; Minieri, M.; Di Nardo, P.; Teodori, L. Dietary Flaxseed Mitigates Impaired Skeletal Muscle Regeneration: In Vivo, in Vitro and in Silico Studies. Int. J. Med. Sci. 2016, 13, 206–219. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Lee, J.H.; Kim, W.J.; Kim, S.H.; Kim, S.Y.; Kim, H.S.; Kim, T.J. Linoleic Acid Attenuates Denervation-Induced Skeletal Muscle Atrophy in Mice through Regulation of Reactive Oxygen Species-Dependent Signaling. Int. J. Mol. Sci. 2022, 23, 4778. [Google Scholar] [CrossRef]
- Komiya, Y.; Kobayashi, C.; Uchida, N.; Otsu, S.; Tanio, T.; Yokoyama, I.; Nagasao, J.; Arihara, K. Effect of Dietary Fish Oil Intake on Ubiquitin Ligase Expression during Muscle Atrophy Induced by Sciatic Nerve Denervation in Mice. Anim. Sci. J. 2019, 90, 1018–1025. [Google Scholar] [CrossRef]
- Jeromson, S.; Mackenzie, I.; Doherty, M.K.; Whitfield, P.D.; Bell, G.; Dick, J.; Shaw, A.; Rao, F.V.; Ashcroft, S.P.; Philp, A.; et al. Lipid Remodeling and an Altered Membrane-Associated Proteome May Drive the Differential Effects of EPA and DHA Treatment on Skeletal Muscle Glucose Uptake and Protein Accretion. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E605–E619. [Google Scholar] [CrossRef] [Green Version]
- Ochi, E.; Tsuchiya, Y. Eicosapentaenoic Acid (EPA) and Docosahexaneoic Acid (DHA) in Muscle Damage and Function. Nutrients 2018, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Han, Y.; Xu, J.; Huang, W.; Li, Z. Medium Chain Triglycerides Enhances Exercise Endurance through the Increased Mitochondrial Biogenesis and Metabolism. PLoS ONE 2018, 13, e0191182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, S.; Ezaki, O.; Suzuki, M. Medium-Chain Triglycerides (8:0 and 10:0) Are Promising Nutrients for Sarcopenia: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2019, 110, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Ezaki, O.; Suzuki, M. Medium-Chain Triglycerides in Combination with Leucine and Vitamin D Increase Muscle Strength and Function in Frail Elderly Adults in a Randomized Controlled Trial. J. Nutr. 2016, 146, 1017–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyagawa, Y.; Mori, T.; Goto, K.; Kawahara, I.; Fujiwara-Tani, R.; Kishi, S.; Sasaki, T.; Fujii, K.; Ohmori, H.; Kuniyasu, H. Intake of Medium-Chain Fatty Acids Induces Myocardial Oxidative Stress and Atrophy. Lipids Health Dis. 2018, 17, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Fujikura, Y.; Kimura, K.; Yamanouchi, K.; Sugihara, H.; Hatakeyama, M.; Zhuang, H.; Abe, T.; Daimon, M.; Morita, H.; Komuro, I.; et al. A Medium-Chain Triglyceride Containing Ketogenic Diet Exacerbates Cardiomyopathy in a CRISPR/Cas9 Gene-Edited Rat Model with Duchenne Muscular Dystrophy. Sci. Rep. 2022, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, S.; Yahata, N.; Saitoh, K.; Yoshimura, T.; Wang, Y.; Taniyama, S.; Nikawa, T.; Tachibana, K.; Hirasaka, K. Dietary Supplementation with Alkylresorcinols Prevents Muscle Atrophy through a Shift of Energy Supply. J. Nutr. Biochem. 2018, 61, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Siva, N. Can Ketogenic Diet Slow Progression of ALS? Lancet Neurol. 2006, 5, 476. [Google Scholar] [CrossRef]
- White, Z.; Theret, M.; Milad, N.; Tung, L.W.; Chen, W.W.H.; Sirois, M.G.; Rossi, F.; Bernatchez, P. Cholesterol Absorption Blocker Ezetimibe Prevents Muscle Wasting in Severe Dysferlin-Deficient and Mdx Mice. J. Cachexia. Sarcopenia Muscle 2022, 13, 544–560. [Google Scholar] [CrossRef]
- Reggio, A.; Rosina, M.; Krahmer, N.; Palma, A.; Petrilli, L.L.; Maiolatesi, G.; Massacci, G.; Salvatori, I.; Valle, C.; Testa, S.; et al. Metabolic Reprogramming of Fibro/Adipogenic Progenitors Facilitates Muscle Regeneration. Life Sci. Alliance 2020, 3, e202000646. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Quan, H.; Kang, S.G.; Huang, K.; Tong, T. Nutraceuticals in the Prevention and Treatment of the Muscle Atrophy. Nutrition 2021, 13, 1914. [Google Scholar] [CrossRef] [PubMed]
- Zhiyin, L.; Jinliang, C.; Qiunan, C.; Yunfei, Y.; Qian, X. Fucoxanthin Rescues Dexamethasone Induced C2C12 Myotubes Atrophy. Biomed. Pharmacother. 2021, 139, 111590. [Google Scholar] [CrossRef]
- Semba, R.D.; Blaum, C.; Guralnik, J.M.; Moncrief, D.T.; Ricks, M.O.; Fried, L.P. Carotenoid and Vitamin E Status Are Associated with Indicators of Sarcopenia among Older Women Living in the Community. Aging Clin. Exp. Res. 2003, 15, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Kariya, Y.; Kitakaze, T.; Yamaji, R.; Harada, N.; Sakamoto, T.; Hosotani, K.; Nakano, Y.; Inui, H. The Preventive Effect of β-Carotene on Denervation-Induced Soleus Muscle Atrophy in Mice. Br. J. Nutr. 2013, 109, 1349–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, L.N.; Hayhoe, R.P.G.; Mulligan, A.A.; Luben, R.N.; Khaw, K.T.; Welch, A.A. Lower Dietary and Circulating Vitamin C in Middle- and Older-Aged Men and Women Are Associated with Lower Estimated Skeletal Muscle Mass. J. Nutr. 2020, 150, 2789–2798. [Google Scholar] [CrossRef]
- Takisawa, S.; Funakoshi, T.; Yatsu, T.; Nagata, K.; Aigaki, T.; Machida, S.; Ishigami, A. Vitamin C Deficiency Causes Muscle Atrophy and a Deterioration in Physical Performance. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gunton, J.E.; Girgis, C.M. Vitamin D and Muscle. Bone Rep. 2018, 8, 163–167. [Google Scholar] [CrossRef]
- Zhu, K.; Austin, N.; Devine, A.; Bruce, D.; Prince, R.L. A Randomized Controlled Trial of the Effects of Vitamin D on Muscle Strength and Mobility in Older Women with Vitamin D Insufficiency. J. Am. Geriatr. Soc. 2010, 58, 2063–2068. [Google Scholar] [CrossRef]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.T.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a Vitamin D and Leucine-Enriched Whey Protein Nutritional Supplement on Measures of Sarcopenia in Older Adults, the PROVIDE Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef]
- Muir, S.W.; Montero-Odasso, M. Effect of Vitamin D Supplementation on Muscle Strength, Gait and Balance in Older Adults: A Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2011, 59, 2291–2300. [Google Scholar] [CrossRef]
- Uusi-Rasi, K.; Patil, R.; Karinkanta, S.; Kannus, P.; Tokola, K.; Lamberg-Allardt, C.; Sievänen, H. Exercise and Vitamin D in Fall Prevention among Older Women: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The Roles of Vitamin D in Skeletal Muscle: Form, Function, and Metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, M.; Seelaender, M.; Sotiropoulos, A.; Coletti, D.; Lancha, A.H. Vitamin D, Muscle Recovery, Sarcopenia, Cachexia, and Muscle Atrophy. Nutrition 2019, 60, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Trojsi, F.; Siciliano, M.; Passaniti, C.; Bisecco, A.; Russo, A.; Lavorgna, L.; Esposito, S.; Ricciardi, D.; Monsurrò, M.R.; Tedeschi, G.; et al. Vitamin D Supplementation Has No Effects on Progression of Motor Dysfunction in Amyotrophic Lateral Sclerosis (ALS). Eur. J. Clin. Nutr. 2020, 74, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Blasco, H.; Madji Hounoum, B.; Dufour-Rainfray, D.; Patin, F.; Maillot, F.; Beltran, S.; Gordon, P.H.; Andres, C.R.; Corcia, P. Vitamin D Is Not a Protective Factor in ALS. CNS Neurosci. Ther. 2015, 21, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Park, J.S.; Oh, K.W.; Oh, S.I.; Park, H.M.; Kim, S.H. Vitamin D Levels Are Not Predictors of Survival in a Clinic Population of Patients with ALS. J. Neurol. Sci. 2016, 367, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Lanznaster, D.; Bejan-Angoulvant, T.; Gandía, J.; Blasco, H.; Corcia, P. Is There a Role for Vitamin D in Amyotrophic Lateral Sclerosis? A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Bo, Y.; Liu, C.; Ji, Z.; Yang, R.; An, Q.; Zhang, X.; You, J.; Duan, D.; Sun, Y.; Zhu, Y.; et al. A High Whey Protein, Vitamin D and E Supplement Preserves Muscle Mass, Strength, and Quality of Life in Sarcopenic Older Adults: A Double-Blind Randomized Controlled Trial. Clin. Nutr. 2019, 38, 159–164. [Google Scholar] [CrossRef]
- Goncharova, P.S.; Davydova, T.K.; Popova, T.E.; Novitsky, M.A.; Petrova, M.M.; Gavrilyuk, O.A.; Al-Zamil, M.; Zhukova, N.G.; Nasyrova, R.F.; Shnayder, N.A. Nutrient Effects on Motor Neurons and the Risk of Amyotrophic Lateral Sclerosis. Nutrition 2021, 13, 3804. [Google Scholar] [CrossRef]
- Kim, C.; Hwang, J.K. Flavonoids: Nutraceutical Potential for Counteracting Muscle Atrophy. Food Sci. Biotechnol. 2020, 29, 1619–1640. [Google Scholar] [CrossRef]
- Salucci, S.; Falcieri, E. Polyphenols and Their Potential Role in Preventing Skeletal Muscle Atrophy. Nutr. Res. 2020, 74, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, A.; Rojasawasthien, T.; Hitomi, S.; Aoki, Y.; Urata, M.; Inoue, A.; Matsubara, T.; Morikawa, K.; Habu, M.; Tominaga, K.; et al. Oral Administration of Geranylgeraniol Rescues Denervation-Induced Muscle Atrophy via Suppression of Atrogin-1. In Vivo 2020, 34, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Urata, M.; Nakajima, T.; Fukuzaki, M.; Masuda, R.; Yoshimoto, Y.; Addison, W.N.; Nakatomi, C.; Morikawa, K.; Zhang, M.; et al. Geranylgeraniol-Induced Myogenic Differentiation of C2C12 Cells. In Vivo 2018, 32, 1427–1432. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, Y.; Ho, H.J.; Yamagishi, M.; Ikemoto, H.; Komai, M.; Shirakawa, H. Cysteine Sulfoxides Enhance Steroid Hormone Production via Activation of the Protein Kinase A Pathway in Testis-Derived I-10 Tumor Cells. Molecules 2020, 25, 4694. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ruegg, U.T.; Kudo, A.; Miyagoe-Suzuki, Y.; Takeda, S. Activation of Calcium Signaling through Trpv1 by NNOS and Peroxynitrite as a Key Trigger of Skeletal Muscle Hypertrophy. Nat. Med. 2013, 19, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Swiderski, K.; Murphy, K.T.; Lynch, G.S. Role for Plant-Derived Antioxidants in Attenuating Cancer Cachexia. Antioxidants 2022, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Novak, V.; Rogelj, B.; Župunski, V. Therapeutic Potential of Polyphenols in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Antioxidants 2021, 10, 1328. [Google Scholar] [CrossRef]
- Hirasaka, K.; Maeda, T.; Ikeda, C.; Haruna, M.; Kohno, S.; Abe, T.; Ochi, A.; Mukai, R.; Oarada, M.; Teshima-Kondo, S.; et al. Isoflavones Derived from Soy Beans Prevent MuRF1-Mediated Muscle Atrophy in C2C12 Myotubes through SIRT1 Activation. J. Nutr. Sci. Vitaminol. 2013, 59, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Tabata, S.; Aizawa, M.; Kinoshita, M.; Ito, Y.; Kawamura, Y.; Takebe, M.; Pan, W.; Sakuma, K. The Influence of Isoflavone for Denervation-Induced Muscle Atrophy. Eur. J. Nutr. 2019, 58, 291–300. [Google Scholar] [CrossRef]
- Shen, L.R.; Parnell, L.D.; Ordovas, J.M.; Lai, C.Q. Curcumin and Aging. Biofactors 2013, 39, 133–140. [Google Scholar] [CrossRef]
- Shen, L.R.; Xiao, F.; Yuan, P.; Chen, Y.; Gao, Q.K.; Parnell, L.D.; Meydani, M.; Ordovas, J.M.; Li, D.; Lai, C.Q. Curcumin-Supplemented Diets Increase Superoxide Dismutase Activity and Mean Lifespan in Drosophila. Age 2013, 35, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Gorza, L.; Germinario, E.; Tibaudo, L.; Vitadello, M.; Tusa, C.; Guerra, I.; Bondì, M.; Salmaso, S.; Caliceti, P.; Vitiello, L.; et al. Chronic Systemic Curcumin Administration Antagonizes Murine Sarcopenia and Presarcopenia. Int. J. Mol. Sci. 2021, 22, 11789. [Google Scholar] [CrossRef] [PubMed]
- Varma, K.; Amalraj, A.; Divya, C.; Gopi, S. The Efficacy of the Novel Bioavailable Curcumin (Cureit) in the Management of Sarcopenia in Healthy Elderly Subjects: A Randomized, Placebo-Controlled, Double-Blind Clinical Study. J. Med. Food 2021, 24, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative Stress: Role of Physical Exercise and Antioxidant Nutraceuticals in Adulthood and Aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.; Sato, H.; Okuda, M.; Wu, J.; Koyama, S.; Izumi, Y.; Waku, T.; Iino, M.; Aoki, M.; Arawaka, S.; et al. Therapeutic Effect of a Novel Curcumin Derivative GT863 on a Mouse Model of Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 2021, 23, 489–495. [Google Scholar] [CrossRef]
- Bhatia, N.K.; Srivastava, A.; Katyal, N.; Jain, N.; Khan, M.A.I.; Kundu, B.; Deep, S. Curcumin Binds to the Pre-Fibrillar Aggregates of Cu/Zn Superoxide Dismutase (SOD1) and Alters Its Amyloidogenic Pathway Resulting in Reduced Cytotoxicity. Biochim. Biophys. Acta 2015, 1854, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Noorafshan, A.; Omidi, A.; Karbalay-Doust, S. Curcumin Protects the Dorsal Root Ganglion and Sciatic Nerve after Crush in Rat. Pathol. Res. Pract. 2011, 207, 577–582. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Yu, H.; Wang, Q.; Chen, Y.; Xiang, L. Curcumin Promotes Nerve Regeneration and Functional Recovery in Rat Model of Nerve Crush Injury. Neurosci. Lett. 2013, 547, 26–31. [Google Scholar] [CrossRef]
- Mohseni, M.; Sahebkar, A.; Askari, G.; Johnston, T.P.; Alikiaii, B.; Bagherniya, M. The Clinical Use of Curcumin on Neurological Disorders: An Updated Systematic Review of Clinical Trials. Phytother. Res. 2021, 35, 6862–6882. [Google Scholar] [CrossRef]
- Shadfar, S.; Couch, M.E.; McKinney, K.A.; Weinstein, L.J.; Yin, X.; Rodriguez, J.E.; Guttridge, D.C.; Willis, M. Oral Resveratrol Therapy Inhibits Cancer-Induced Skeletal Muscle and Cardiac Atrophy in Vivo. Nutr. Cancer 2011, 63, 749–762. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.J.; Sun, Y.N.; Chen, S.J.; Liu, S.; Jiang, G.R. Resveratrol Attenuates Skeletal Muscle Atrophy Induced by Chronic Kidney Disease via MuRF1 Signaling Pathway. Biochem. Biophys. Res. Commun. 2017, 487, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol Prevents Sarcopenic Obesity by Reversing Mitochondrial Dysfunction and Oxidative Stress via the PKA/LKB1/AMPK Pathway. Aging 2019, 11, 2217–2240. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, H.; Song, G.; Yang, Y.; Zou, X.; Han, P.; Li, S. Resveratrol Improves Muscle Atrophy by Modulating Mitochondrial Quality Control in STZ-Induced Diabetic Mice. Mol. Nutr. Food Res. 2018, 62, 1700941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asami, Y.; Aizawa, M.; Kinoshita, M.; Ishikawa, J.; Sakuma, K. Resveratrol Attenuates Denervation-Induced Muscle Atrophy Due to the Blockade of Atrogin-1 and P62 Accumulation. Int. J. Med. Sci. 2018, 15, 628–637. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, J.; Liu, Y.; Huang, D.; Lu, L. Resveratrol Regulates the Recovery of Rat Sciatic Nerve Crush Injury by Promoting the Autophagy of Schwann Cells. Life Sci. 2020, 256, 117959. [Google Scholar] [CrossRef]
- Lançon, A.; Kaminski, J.; Tili, E.; Michaille, J.J.; Latruffe, N. Control of MicroRNA Expression as a New Way for Resveratrol to Deliver Its Beneficial Effects. J. Agric. Food Chem. 2012, 60, 8783–8789. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, L.; Li, X.; Liu, J.; Li, H.; Gong, L.; Zhang, J.; Wang, J.; Sun, B. The Progress of Nomenclature, Structure, Metabolism, and Bioactivities of Oat Novel Phytochemical: Avenanthramides. J. Agric. Food Chem. 2022, 70, 446–457. [Google Scholar] [CrossRef]
- Yeo, D.; Kang, C.; Zhang, T.; Ji, L.L. Avenanthramides Attenuate Inflammation and Atrophy in Muscle Cells. J. Sport Health Sci. 2019, 8, 189. [Google Scholar] [CrossRef]
- Sur, R.; Nigam, A.; Grote, D.; Liebel, F.; Southall, M.D. Avenanthramides, Polyphenols from Oats, Exhibit Anti-Inflammatory and Anti-Itch Activity. Arch. Dermatol. Res. 2008, 300, 569–574. [Google Scholar] [CrossRef]
- Guo, W.; Wise, M.L.; Collins, F.W.; Meydani, M. Avenanthramides, Polyphenols from Oats, Inhibit IL-1β-Induced NF-ΚB Activation in Endothelial Cells. Free Radic. Biol. Med. 2008, 44, 415–429. [Google Scholar] [CrossRef]
- Kang, C.; Shin, W.S.; Yeo, D.; Lim, W.; Ji, L.L. Anti-Inflammatory Effect of Avenanthramides via NF-ΚB Pathways in C2C12 Skeletal Muscle Cells. Free Radic. Biol. Med. 2018, 117, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, C.S.; Joe, Y.; Chung, H.T.; Ha, T.Y.; Yu, R. Quercetin Reduces Tumor Necrosis Factor Alpha-Induced Muscle Atrophy by Upregulation of Heme Oxygenase-1. J. Med. Food 2018, 21, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Matsui, N.; Fujikura, Y.; Matsumoto, N.; Hou, D.X.; Kanzaki, N.; Shibata, H.; Horikawa, M.; Iwasa, K.; Hirasaka, K.; et al. Preventive Effect of Dietary Quercetin on Disuse Muscle Atrophy by Targeting Mitochondria in Denervated Mice. J. Nutr. Biochem. 2016, 31, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.B.; Lee, H.S.; Kim, D.H.; Moon, J.M.; Park, Y. Tannase-Converted Green Tea Extract with High (-)-Epicatechin Inhibits Skeletal Muscle Mass in Aged Mice. Evid. Based. Complement. Alternat. Med. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meador, B.M.; Mirza, K.A.; Tian, M.; Skelding, M.B.; Reaves, L.A.; Edens, N.K.; Tisdale, M.J.; Pereira, S.L. The Green Tea Polyphenol Epigallocatechin-3-Gallate (EGCg) Attenuates Skeletal Muscle Atrophy in a Rat Model of Sarcopenia. J. Frailty Aging 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Choi, W.H.; Jang, Y.J.; Son, H.J.; Ahn, J.; Jung, C.H.; Ha, T.Y. Apigenin Inhibits Sciatic Nerve Denervation–Induced Muscle Atrophy. Muscle Nerve 2018, 58, 314–318. [Google Scholar] [CrossRef]
- Aoyama, S.; Jia, H.; Nakazawa, K.; Yamamura, J.; Saito, K.; Kato, H. Dietary Genistein Prevents Denervation-Induced Muscle Atrophy in Male Rodents via Effects on Estrogen Receptor-α. J. Nutr. 2016, 146, 1147–1154. [Google Scholar] [CrossRef] [Green Version]
- Mukai, R.; Horikawa, H.; Fujikura, Y.; Kawamura, T.; Nemoto, H.; Nikawa, T.; Terao, J. Prevention of Disuse Muscle Atrophy by Dietary Ingestion of 8-Prenylnaringenin in Denervated Mice. PLoS ONE 2012, 7, e45048. [Google Scholar] [CrossRef] [Green Version]
- Dyle, M.C.; Ebert, S.M.; Cook, D.P.; Kunkel, S.D.; Fox, D.K.; Bongers, K.S.; Bullard, S.A.; Dierdorff, J.M.; Adams, C.M. Systems-Based Discovery of Tomatidine as a Natural Small Molecule Inhibitor of Skeletal Muscle Atrophy. J. Biol. Chem. 2014, 289, 14913–14924. [Google Scholar] [CrossRef] [Green Version]
- Fang, E.F.; Waltz, T.B.; Kassahun, H.; Lu, Q.; Kerr, J.S.; Morevati, M.; Fivenson, E.M.; Wollman, B.N.; Marosi, K.; Wilson, M.A.; et al. Tomatidine Enhances Lifespan and Healthspan in C. Elegans through Mitophagy Induction via the SKN-1/Nrf2 Pathway. Sci. Rep. 2017, 7, 46208. [Google Scholar] [CrossRef] [Green Version]
- Ilaiyaraja, N.; Khanum, F. Antioxidant Potential of Tinospora Cordifolia Extracts and Their Protective Effect on Oxidation of Biomolecules. Pharmacogn. J. 2011, 3, 56–62. [Google Scholar] [CrossRef]
- Sharma, B.; Dutt, V.; Kaur, N.; Mittal, A.; Dabur, R. Tinospora Cordifolia Protects from Skeletal Muscle Atrophy by Alleviating Oxidative Stress and Inflammation Induced by Sciatic Denervation. J. Ethnopharmacol. 2020, 254, 112720. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Zheng, Z.; Zhang, X.; Wei, Y.; Chu, K.; Brown, J.; Hong, G.; Chen, L. Salidroside-Mediated Neuroprotection Is Associated with Induction of Early Growth Response Genes (Egrs) Across a Wide Therapeutic Window. Neurotox. Res. 2015, 28, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xun, L.; Jin, G.; Shi, L. Salidroside Protects Renal Tubular Epithelial Cells from Hypoxia/Reoxygenation Injury in Vitro. J. Pharmacol. Sci. 2018, 137, 170–176. [Google Scholar] [CrossRef]
- Wu, C.; Tang, L.; Ni, X.; Xu, T.; Fang, Q.; Xu, L.; Ma, W.; Yang, X.; Sun, H. Salidroside Attenuates Denervation-Induced Skeletal Muscle Atrophy through Negative Regulation of pro-Inflammatory Cytokine. Front. Physiol. 2019, 10, 665. [Google Scholar] [CrossRef] [Green Version]
- Chico, L.; Ienco, E.C.; Bisordi, C.; Lo Gerfo, A.; Petrozzi, L.; Petrucci, A.; Mancuso, M.; Siciliano, G. Amyotrophic Lateral Sclerosis and Oxidative Stress: A Double-Blind Therapeutic Trial After Curcumin Supplementation. CNS Neurol. Disord. Drug Targets 2018, 17, 767–779. [Google Scholar] [CrossRef]
- Costa, R.G.F.; Caro, P.L.; de Matos-Neto, E.M.; Lima, J.D.C.C.; Radloff, K.; Alves, M.J.; Camargo, R.G.; Pessoa, A.F.M.; Simoes, E.; Gama, P.; et al. Cancer Cachexia Induces Morphological and Inflammatory Changes in the Intestinal Mucosa. J. Cachexia. Sarcopenia Muscle 2019, 10, 1116–1127. [Google Scholar] [CrossRef] [Green Version]
- Mangiola, F.; Nicoletti, A.; Gasbarrini, A.; Ponziani, F.R. Gut Microbiota and Aging. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7404–7413. [Google Scholar] [CrossRef]
- Ubachs, J.; Ziemons, J.; Soons, Z.; Aarnoutse, R.; van Dijk, D.P.J.; Penders, J.; van Helvoort, A.; Smidt, M.L.; Kruitwagen, R.F.P.M.; Baade-Corpelijn, L.; et al. Gut Microbiota and Short-Chain Fatty Acid Alterations in Cachectic Cancer Patients. J. Cachexia. Sarcopenia Muscle 2021, 12, 2007–2021. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M. Muscle Wasting: The Gut Microbiota as a New Therapeutic Target? Int. J. Biochem. Cell Biol. 2013, 45, 2186–2190. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The Gut Microbiota Influences Skeletal Muscle Mass and Function in Mice. Sci. Transl. Med. 2019, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus Plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrition 2016, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrition 2019, 11, 1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giron, M.; Thomas, M.; Dardevet, D.; Chassard, C.; Savary-Auzeloux, I. Gut Microbes and Muscle Function: Can Probiotics Make Our Muscles Stronger? J. Cachexia. Sarcopenia Muscle 2022, 13, 1460–1476. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Y.; Yu, X. A Narrative Review of Gut-Muscle Axis and Sarcopenia: The Potential Role of Gut Microbiota. Int. J. Gen. Med. 2021, 14, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Nehmi, V.A.; Murata, G.M.; de Moraes, R.C.M.; Lima, G.C.A.; De Miranda, D.A.; Radloff, K.; Costa, R.G.F.; de Jesus, J.d.C.R.; De Freitas, J.A.; Viana, N.I.; et al. A Novel Supplement with Yeast β-Glucan, Prebiotic, Minerals and Silybum Marianum Synergistically Modulates Metabolic and Inflammatory Pathways and Improves Steatosis in Obese Mice. J. Integr. Med. 2021, 19, 439–450. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’sullivan, O.; et al. Gut Microbiota Composition Correlates with Diet and Health in the Elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Montiel-Rojas, D.; Nilsson, A.; Santoro, A.; Franceschi, C.; Bazzocchi, A.; Battista, G.; de Groot, L.C.P.G.M.; Feskens, E.J.M.; Berendsen, A.; Pietruszka, B.; et al. Dietary Fibre May Mitigate Sarcopenia Risk: Findings from the NU-AGE Cohort of Older European Adults. Nutrients 2020, 12, 1075. [Google Scholar] [CrossRef] [Green Version]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Higher Dietary Fibre Intake Is Associated with Increased Skeletal Muscle Mass and Strength in Adults Aged 40 Years and Older. J. Cachexia. Sarcopenia Muscle 2021, 12, 2134–2144. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary Fiber Intervention on Gut Microbiota Composition in Healthy Adults: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Muegge, B.D.; Kuczynski, J.; Knights, D.; Clemente, J.C.; González, A.; Fontana, L.; Henrissat, B.; Knight, R.; Gordon, J.I. Diet Drives Convergence in Gut Microbiome Functions across Mammalian Phylogeny and within Humans. Science 2011, 332, 970–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-Chain Fatty Acids as Potential Regulators of Skeletal Muscle Metabolism and Function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Li, S.; Guerin-Deremaux, L.; Pochat, M.; Wils, D.; Reifer, C.; Miller, L.E. NUTRIOSE Dietary Fiber Supplementation Improves Insulin Resistance and Determinants of Metabolic Syndrome in Overweight Men: A Double-Blind, Randomized, Placebo-Controlled Study. Appl. Physiol. Nutr. Metab. 2010, 35, 773–782. [Google Scholar] [CrossRef]
- Solà, R.; Bruckert, E.; Valls, R.M.; Narejos, S.; Luque, X.; Castro-Cabezas, M.; Doménech, G.; Torres, F.; Heras, M.; Farrés, X.; et al. Soluble Fibre (Plantago Ovata Husk) Reduces Plasma Low-Density Lipoprotein (LDL) Cholesterol, Triglycerides, Insulin, Oxidised LDL and Systolic Blood Pressure in Hypercholesterolaemic Patients: A Randomised Trial. Atherosclerosis 2010, 211, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Aliasgharzadeh, A.; Dehghan, P.; Gargari, B.P.; Asghari-Jafarabadi, M. Resistant Dextrin, as a Prebiotic, Improves Insulin Resistance and Inflammation in Women with Type 2 Diabetes: A Randomised Controlled Clinical Trial. Br. J. Nutr. 2015, 113, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Dehghan, P.; Gargari, B.P.; Jafar-Abadi, M.A.; Aliasgharzadeh, A. Inulin Controls Inflammation and Metabolic Endotoxemia in Women with Type 2 Diabetes Mellitus: A Randomized-Controlled Clinical Trial. Int. J. Food Sci. Nutr. 2014, 65, 117–123. [Google Scholar] [CrossRef]
- Buigues, C.; Fernández-Garrido, J.; Pruimboom, L.; Hoogland, A.J.; Navarro-Martínez, R.; Martínez-Martínez, M.; Verdejo, Y.; Carmen Mascarós, M.; Peris, C.; Cauli, O. Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial. Int. J. Mol. Sci. 2016, 17, 932. [Google Scholar] [CrossRef] [Green Version]
- Di Gioia, D.; Bozzi Cionci, N.; Baffoni, L.; Amoruso, A.; Pane, M.; Mogna, L.; Gaggìa, F.; Lucenti, M.A.; Bersano, E.; Cantello, R.; et al. A Prospective Longitudinal Study on the Microbiota Composition in Amyotrophic Lateral Sclerosis. BMC Med. 2020, 18, 153. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Wang, J.; Sun, B. MicroRNAs: The Novel Mediators for Nutrient-Modulating Biological Functions. Trends Food Sci. Technol. 2021, 114, 167–175. [Google Scholar] [CrossRef]
- Cannataro, R.; Carbone, L.; Petro, J.L.; Cione, E.; Vargas, S.; Angulo, H.; Forero, D.A.; Odriozola-Martínez, A.; Kreider, R.B.; Bonilla, D.A. Sarcopenia: Etiology, Nutritional Approaches, and MiRNAs. Int. J. Mol. Sci. 2021, 22, 9724. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moresi, V.; Renzini, A.; Cavioli, G.; Seelaender, M.; Coletti, D.; Gigli, G.; Cedola, A. Functional Nutrients to Ameliorate Neurogenic Muscle Atrophy. Metabolites 2022, 12, 1149. https://doi.org/10.3390/metabo12111149
Moresi V, Renzini A, Cavioli G, Seelaender M, Coletti D, Gigli G, Cedola A. Functional Nutrients to Ameliorate Neurogenic Muscle Atrophy. Metabolites. 2022; 12(11):1149. https://doi.org/10.3390/metabo12111149
Chicago/Turabian StyleMoresi, Viviana, Alessandra Renzini, Giorgia Cavioli, Marilia Seelaender, Dario Coletti, Giuseppe Gigli, and Alessia Cedola. 2022. "Functional Nutrients to Ameliorate Neurogenic Muscle Atrophy" Metabolites 12, no. 11: 1149. https://doi.org/10.3390/metabo12111149