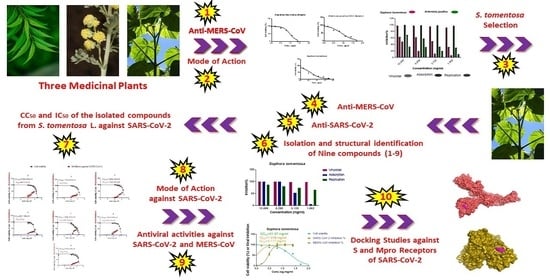
Investigating the Potential Anti-SARS-CoV-2 and Anti-MERS-CoV Activities of Yellow Necklacepod among Three Selected Medicinal Plants: Extraction, Isolation, Identification, In Vitro, Modes of Action, and Molecular Docking Studies
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Investigation
Identification of Isolated Compounds from S. tomentosa
2.2. Biological Activity Evaluations
2.2.1. Antiviral Activity for Three Medicinal Plants against MERS-CoV by Plaque Reduction Assay
2.2.2. Mode of Action against MERS-CoV
2.2.3. Comparison between the Antiviral Activity of S. tomentosa against MERS-CoV and SARS-CoV-2
2.2.4. Antiviral Activity of the Isolated and Identified Compounds from S. tomentosa L. against SARS-CoV-2 by Crystal Violet Assay
2.2.5. Mode of Action of S. tomentosa L. against SARS-CoV-2
2.2.6. Antiviral Activities for Crude S. tomentosa L. against Both SARS-CoV-2 and MERS-CoV by Crystal Violet Assay
2.3. Docking Studies
- (a)
- Compound 4 was stabilized inside the S binding pocket through the formation of one H-bond with crucial amino acid Asp80 and with a binding score of −5.71 kcal/mol. This indicates the large binding affinity of the mentioned compound which does not need more binding sites and has an expected superior intrinsic activity as well.
- (b)
- On the other hand, compound 8 showed the formation of two H-bonds with Asp80 and Asn137 amino acids. Its binding score was found to be −7.03 kcal/mol.
- (a)
- The docked O6K inhibitor of the dimeric Mpr° binding pocket formed three H-bonds with Glu166, Asn142, and Ser1 amino acids. Moreover, it achieved a score of −8.98 kcal/mol.
- (b)
- Notably, compound 4 bound the crucial Glu166 amino acid with one H-bond which was enough to stabilize itself and produce its inhibitory effect with a binding score of −6.44 kcal/mol.
- (c)
- Furthermore, compound 8 formed three H-bonds with Glu166, Asn142, and Gln192 with a binding score of −7.36 kcal/mol.
3. Materials and Methods
3.1. Plant Materials
3.2. Preparation of Extracts for Antiviral Assays
3.3. Phytochemical Study
3.3.1. General
3.3.2. Material for Chromatography
3.3.3. Chemicals
3.3.4. Solvent Systems and Spray Reagents
3.3.5. Extraction and Isolation
3.4. Virus and Cells
3.5. Biological Activity Evaluations
3.5.1. Determination Titers of Viruses by Plaque Titration Assay
3.5.2. MTT Cytotoxicity Assay (CC50)
3.5.3. Plaque Reduction Assay
3.5.4. Mode of Action of Virus Inhibition
- (i)
- Inhibition of budding and viral replication.
- (ii)
- The ability of each extract to inhibit the attachment of the virus to infected cells—membrane fusion is known to block the viral entry (viral adsorption).
- (iii)
- The direct effect of each extract is to inactivate the virus viability (virucidal activity).
Viral Replication
Viral Adsorption
Virucidal
3.5.5. Inhibitory Concentration 50 (IC50) Calculation
3.6. Docking Studies
3.6.1. Validation of the MOE Program
3.6.2. Preparation of the S. tomentosa Isolated Compounds
3.6.3. Preparation of the S and Mpr° Receptors of SARS-CoV-2
3.6.4. Docking of Each Database into the Corresponding Binding Pocket of SARS-CoV-2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Karmalawy, A.A.; Soltane, R.; Abo Elmaaty, A.; Tantawy, M.A.; Antar, S.A.; Yahya, G.; Chrouda, A.; Pashameah, R.A.; Mustafa, M.; Abu Mraheil, M.; et al. Coronavirus Disease (COVID-19) Control between Drug Repurposing and Vaccination: A Comprehensive Overview. Vaccines 2021, 9, 1317. [Google Scholar] [CrossRef] [PubMed]
- Ashour, N.A.; Elmaaty, A.A.; Sarhan, A.A.; Elkaeed, E.B.; Moussa, A.M.; Erfan, I.A.; Al-Karmalawy, A.A. A Systematic Review of the Global Intervention for SARS-CoV-2 Combating: From Drugs Repurposing to Molnupiravir Approval. Drug Des. Dev. Ther. 2022, 16, 685. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al-Ahmed, S.H.; Haque, S.; Sah, R.; Tiwari, R.; Malik, Y.S.; Dhama, K.; Yatoo, M.I.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. SARS-CoV-2, SARS-CoV, and MERS-COV: A comparative overview. Infez Med 2020, 28, 174–184. [Google Scholar] [PubMed]
- Roshdy, W.H.; Khalifa, M.K.; San, J.E.; Tegally, H.; Wilkinson, E.; Showky, S.; Martin, D.P.; Moir, M.; Naguib, A.; Elguindy, N.; et al. SARS-CoV-2 Genetic Diversity and Lineage Dynamics in Egypt during the First 18 Months of the Pandemic. Viruses 2022, 14, 1878. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, A.A.; Ashour, N.A.; Al-Karmalawy, A.A. The journey of antimalarial drugs against SARS-CoV-2: Review article. Inform. Med. Unlocked 2021, 24, 100604. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E. Epidemiology, virology, and clinical features of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19). Clin. Exp. Pediatr. 2020, 63, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdallah, A.E.; Alesawy, M.S.; Eissa, S.I.; El-Fakharany, E.M.; Kalaba, M.H.; Sharaf, M.H.; Abo Shama, N.M.; Mahmoud, S.H.; Mostafa, A.; Al-Karmalawy, A.A.; et al. Design and synthesis of new 4-(2-nitrophenoxy)benzamide derivatives as potential antiviral agents: Molecular modeling and in vitro antiviral screening. New J. Chem. 2021, 45, 16557–16571. [Google Scholar] [CrossRef]
- Abo Elmaaty, A.; Hamed, M.I.A.; Ismail, M.I.B.; Elkaeed, E.S.; Abulkhair, H.; Khattab, M.; Al-Karmalawy, A.A. Computational Insights on the Potential of Some NSAIDs for Treating COVID-19: Priority Set and Lead Optimization. Molecules 2021, 26, 3772. [Google Scholar] [CrossRef]
- Nascimento Junior, J.A.C.; Santos, A.M.; Quintans-Junior, L.J.; Walker, C.I.B.; Borges, L.P.; Serafini, M.R. SARS, MERS and SARS-CoV-2 (COVID-19) treatment: A patent review. Expert Opin. Ther. Pat. 2020, 30, 567–579. [Google Scholar] [CrossRef]
- Al-Karmalawy, A.A.; Alnajjar, R.; Dahab, M.; Metwaly, A.; Eissa, I. Molecular docking and dynamics simulations reveal the potential of anti-HCV drugs to inhibit COVID-19 main protease. Pharm. Sci. 2021, 27, S109–S121. [Google Scholar] [CrossRef]
- Al-Karmalawy, A.A.; Dahab, M.A.; Metwaly, A.M.; Elhady, S.S.; Elkaeed, E.B.; Eissa, I.H.; Darwish, K.M. Molecular Docking and Dynamics Simulation Revealed the Potential Inhibitory Activity of ACEIs Against SARS-CoV-2 Targeting the h ACE2 Receptor. Front. Chem. 2021, 9, 661230. [Google Scholar] [CrossRef] [PubMed]
- Elmaaty, A.A.; Eldehna, W.M.; Khattab, M.; Kutkat, O.; Alnajjar, R.; El-Taweel, A.N.; Al-Rashood, S.T.; Abourehab, M.A.S.; Binjubair, F.A.; Saleh, M.A.; et al. Anticoagulants as Potential SARS-CoV-2 Mpro Inhibitors for COVID-19 Patients: In Vitro, Molecular Docking, Molecular Dynamics, DFT, and SAR Studies. Int. J. Mol. Sci. 2022, 23, 12235. [Google Scholar] [CrossRef] [PubMed]
- Elagawany, M.; Elmaaty, A.A.; Mostafa, A.; Abo Shama, N.M.; Santali, E.Y.; Elgendy, B.; Al-Karmalawy, A.A. Ligand-based design, synthesis, computational insights, and in vitro studies of novel N-(5-Nitrothiazol-2-yl)-carboxamido derivatives as potent inhibitors of SARS-CoV-2 main protease. J. Enzym. Inhib. Med. Chem. 2022, 37, 2112–2132. [Google Scholar] [CrossRef] [PubMed]
- Kutkat, O.; Moatasim, Y.; Al-Karmalawy, A.A.; Abulkhair, H.S.; Gomaa, M.R.; El-Taweel, A.N.; Abo Shama, N.M.; GabAllah, M.; Mahmoud, D.B.; Kayali, G.; et al. Robust antiviral activity of commonly prescribed antidepressants against emerging coronaviruses: In vitro and in silico drug repurposing studies. Sci. Rep. 2022, 12, 12920. [Google Scholar] [CrossRef]
- El Gizawy, H.A.; Boshra, S.A.; Mostafa, A.; Mahmoud, S.H.; Ismail, M.I.; Alsfouk, A.A.; Taher, A.T.; Al-Karmalawy, A.A. Pimenta dioica (L.) Merr. Bioactive Constituents Exert Anti-SARS-CoV-2 and Anti-Inflammatory Activities: Molecular Docking and Dynamics, In Vitro, and In Vivo Studies. Molecules 2021, 26, 5844. [Google Scholar] [CrossRef] [PubMed]
- Soltane, R.; Chrouda, A.; Mostafa, A.; Al-Karmalawy, A.A.; Chouaïb, K.; dhahri, A.; Pashameah, R.A.; Alasiri, A.; Kutkat, O.; Shehata, M.; et al. Strong Inhibitory Activity and Action Modes of Synthetic Maslinic Acid Derivative on Highly Pathogenic Coronaviruses: COVID-19 Drug Candidate. Pathogens 2021, 10, 623. [Google Scholar] [CrossRef]
- Abd-Alla, H.I.; Sweelam, H.-t.M.; Mohamed, T.A.; Gabr, M.M.; El-Safty, M.M.; Hegazy, M.-E.F. Efficacy of extracts and iridoid glucosides from Pentas lanceolata on humoral and cell-mediated immune response of viral vaccine. Med. Chem. Res. 2017, 26, 2196–2204. [Google Scholar] [CrossRef]
- Abd-Alla, H.I.; Abu-Gabal, N.S.; Hassan, A.Z.; El-Safty, M.M.; Shalaby, N.M. Antiviral activity of Aloe hijazensis against some haemagglutinating viruses infection and its phytoconstituents. Arch. Pharmacal Res. 2012, 35, 1347–1354. [Google Scholar] [CrossRef]
- Al-Karmalawy, A.A.; Farid, M.M.; Mostafa, A.; Ragheb, A.Y.H.; Mahmoud, S.; Shehata, M.; Shama, N.M.A.; GabAllah, M.; Mostafa-Hedeab, G.; Marzouk, M.M. Naturally Available Flavonoid Aglycones as Potential Antiviral Drug Candidates against SARS-CoV-2. Molecules 2021, 26, 6559. [Google Scholar] [CrossRef]
- Zaki, A.A.; Al-Karmalawy, A.A.; El-Amier, Y.A.; Ashour, A. Molecular docking reveals the potential of Cleome amblyocarpa isolated compounds to inhibit COVID-19 virus main protease. New J. Chem. 2020, 44, 16752–16758. [Google Scholar] [CrossRef]
- Arbab, A.H.; Parvez, M.K.; Al-Dosari, M.S.; Al-Rehaily, A.J. In vitro evaluation of novel antiviral activities of 60 medicinal plants extracts against hepatitis B virus. Exp. Ther. Med. 2017, 14, 626–634. [Google Scholar] [CrossRef] [Green Version]
- Cong, Y.; Gross, R.; Zhou, H.; Frieman, M.; Bollinger, L.; Wada, J.; Hensley, L.E.; Jahrling, P.B.; Dyall, J.; Holbrook, M.R. MERS-CoV pathogenesis and antiviral efficacy of licensed drugs in human monocyte-derived antigen-presenting cells. PloS ONE 2018, 13, e0194868. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.F.; Abd-Alla, H.I.; Ali, S.A.; Aba-Alez, R.; Abu-Krisha, M.; Mamdouh, M.M. Bioinformatics: Inflammatory cytokines and attenuation of diabetes hypercholesterolemia-induced renal injury using morning glory and necklace pod extracts. Asian J. Pharm. Clin. Res. 2017, 10, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Abd-Alla, H.I.; Heba-tollah, M.S.; El-Kashak, W.A.; El-Safty, M.M. Evaluation of immune boosting properties and combating of multiple respiratory viral infections by fifteen Euphorbiaceae plant extracts. Pharmacogn. J. 2019, 11, 1490–1503. [Google Scholar] [CrossRef] [Green Version]
- Abd-Alla, H.I.; Ibrahim, M.T.; Taie, H.A.A.; Hasan, M.A.; Shalaby, N.M. Antioxidant and the Efficacy of Sophora secundiflora and Methoxyisoflavones in the Immune Function of Pigeons Vaccinated against Paramyxovirus Serotype-1. Pharmacogn. J. 2020, 12, 1276–1288. [Google Scholar] [CrossRef]
- Kshirsagar, S.G.; Rao, R.V. Antiviral and immunomodulation effects of Artemisia. Medicina 2021, 57, 217. [Google Scholar] [CrossRef]
- Atawodi, S.E.; Atawodi, J.C. Azadirachta indica (neem): A plant of multiple biological and pharmacological activities. Phytochem. Rev. 2009, 8, 601–620. [Google Scholar] [CrossRef]
- Wafaa, A.; Howaida, I.; Hassan, A.; El-Safty, M. Chemical composition and’in vitro’antiviral activity of Azadirachta indica A. Juss (neem) leaves and fruits against newcastle disease virus and infectious bursal disease virus. Aust. J. Basic Appl. Sci. 2007, 1, 801–812. [Google Scholar]
- Aziz, W.M.; Hamed, M.A.; Abd-Alla, H.I.; Ahmed, S.A. Pulicaria crispa mitigates nephrotoxicity induced by carbon tetrachloride in rats via regulation oxidative, inflammatory, tubular and glomerular indices. Biomarkers 2022, 27, 35–43. [Google Scholar] [CrossRef]
- Yu, M.-S.; Lee, J.; Lee, J.M.; Kim, Y.; Chin, Y.-W.; Jee, J.-G.; Keum, Y.-S.; Jeong, Y.-J. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorganic Med. Chem. Lett. 2012, 22, 4049–4054. [Google Scholar] [CrossRef]
- Awad, H.M.; Abd-Alla, H.I.; Mahmoud, K.H.; El-Toumy, S.A. In vitro anti-nitrosative, antioxidant, and cytotoxicity activities of plant flavonoids: A comparative study. Med. Chem. Res. 2014, 23, 3298–3307. [Google Scholar] [CrossRef]
- Guo, G.; Ye, L.; Pan, K.; Chen, Y.; Xing, D.; Yan, K.; Chen, Z.; Ding, N.; Li, W.; Huang, H. New insights of emerging SARS-CoV-2: Epidemiology, etiology, clinical features, clinical treatment, and prevention. Front. Cell Dev. Biol. 2020, 8, 410. [Google Scholar] [CrossRef]
- Elebeedy, D.; Elkhatib, W.F.; Kandeil, A.; Ghanem, A.; Kutkat, O.; Alnajjar, R.; Saleh, M.A.; Abd El Maksoud, A.I.; Badawy, I.; Al-Karmalawy, A.A. Anti-SARS-CoV-2 activities of tanshinone IIA, carnosic acid, rosmarinic acid, salvianolic acid, baicalein, and glycyrrhetinic acid between computational and in vitro insights. RSC Adv. 2021, 11, 29267–29286. [Google Scholar] [CrossRef]
- El-Shershaby, M.H.; Ghiaty, A.; Bayoumi, A.H.; Al-Karmalawy, A.A.; Husseiny, E.M.; El-Zoghbi, M.S.; Abulkhair, H.S. From triazolophthalazines to triazoloquinazolines: A bioisosterism-guided approach toward the identification of novel PCAF inhibitors with potential anticancer activity. Bioorganic Med. Chem. 2021, 42, 116266. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Mostafa, A.; Al-Karmalawy, A.A.; Zidan, A.; Abulkhair, H.S.; Mahmoud, S.H.; Shehata, M.; Elhefnawi, M.M.; Ali, M.A. Telaprevir is a potential drug for repurposing against SARS-CoV-2: Computational and <em>in vitro</em> studies. Heliyon 2021, 7, E07962. [Google Scholar] [CrossRef]
- Abd-Alla, H.I.; Hassan, A.Z.; Soltan, M.M.; Abdelwahab, A.B.; Hanna, A.G. Potential protein antiglycation, antiproliferation, and in silico study on the antidiabetic enzymes of bioactive metabolites from Adonis microcarpa DC and their ADMET properties. J. Appl. Pharm. Sci. 2021, 12, 106–119. [Google Scholar]
- Soltan, M.A.; Elbassiouny, N.; Gamal, H.; Elkaeed, E.B.; Eid, R.A.; Eldeen, M.A.; Al-Karmalawy, A.A. In Silico Prediction of a Multitope Vaccine against Moraxella catarrhalis: Reverse Vaccinology and Immunoinformatics. Vaccines 2021, 9, 669. [Google Scholar] [CrossRef]
- El-Shershaby, M.H.; El-Gamal, K.M.; Bayoumi, A.H.; El-Adl, K.; Alswah, M.; Ahmed, H.E.A.; Al-Karmalamy, A.A.; Abulkhair, H.S. The antimicrobial potential and pharmacokinetic profiles of novel quinoline-based scaffolds: Synthesis and in silico mechanistic studies as dual DNA gyrase and DHFR inhibitors. New J. Chem. 2021, 45, 13986–14004. [Google Scholar] [CrossRef]
- Abd-Alla, H.I.; Soltan, M.M.; Hassan, A.Z.; Taie, H.A.; Abo-Salem, H.M.; Karam, E.A.; El-Safty, M.M.; Hanna, A.G. Cardenolides and pentacyclic triterpenes isolated from Acokanthera oblongifolia leaves: Their biological activities with molecular docking study. Z. Für Nat. C 2021, 76, 301–315. [Google Scholar] [CrossRef]
- Norrehed, S.; Johansson, H.; Grennberg, H.; Gogoll, A. Improved stereochemical analysis of conformationally flexible diamines by binding to a bisporphyrin molecular clip. Chem. –A Eur. J. 2013, 19, 14631–14638. [Google Scholar] [CrossRef]
- Duddeck, H.; Kaiser, M. 13C NMR spectroscopy of coumarin derivatives. Org. Magn. Reson. 1982, 20, 55–72. [Google Scholar] [CrossRef]
- Shalaby, N.M.; Abd-Alla, H.I.; Aly, H.F.; Albalawy, M.A.; Shaker, K.H.; Bouajila, J. Preliminary in vitro and in vivo evaluation of antidiabetic activity of Ducrosia anethifolia Boiss. and its linear furanocoumarins. BioMed Res. Int. 2014, 2014, 480545. [Google Scholar]
- Harborne, J.; Mabry, T. The Flavonoids: Advances in Research; Chapman and Hall Ltd.: New York, NY, USA, 1982. [Google Scholar]
- Agrawal, P.K. Carbon-13 NMR of flavonoids; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Anton, R.; Jiang, Y.; Weniger, B.; Beck, J.; Rivier, L. Pharmacognosy of Mimosa tenuiflora (willd.) poiret. J. Ethnopharmacol. 1993, 38, 145–152. [Google Scholar] [CrossRef]
- Faizi, S.; Ali, M.; Saleem, R.; Bibi, S. Complete 1H and 13C NMR assignments of stigma-5-en-3-O-β-glucoside and its acetyl derivative. Magn. Reson. Chem. 2001, 39, 399–405. [Google Scholar] [CrossRef]
- Quradha, M.M.; Khan, R.; Adhikari, A.; Rauf, A.; Rashid, U.; Bawazeer, S.; Al-Awthan, Y.S.; Bahattab, O.; Mubarak, M.S. Isolation, biological evaluation, and molecular docking studies of compounds from Sophora mollis (Royle) Graham Ex Baker. ACS Omega 2021, 6, 15911–15919. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hayden, F.G.; Cote, K.; Douglas Jr, R.G. Plaque inhibition assay for drug susceptibility testing of influenza viruses. Antimicrob. Agents Chemother. 1980, 17, 865–870. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Ma, G.; Li, N.; Deng, Q.; Yin, Y.; Huang, R. Investigation of in vitro and in vivo antioxidant activities of flavonoids rich extract from the berries of Rhodomyrtus tomentosa (Ait.) Hassk. Food Chem. 2015, 173, 194–202. [Google Scholar] [CrossRef]
- Abd-Alla, H.I.; Souguir, D.; Radwan, M.O. Genus Sophora: A comprehensive review on secondary chemical metabolites and their biological aspects from past achievements to future perspectives. Arch. Pharmacal Res. 2021, 44, 903–986. [Google Scholar] [CrossRef]
- Boozari, M.; Soltani, S.; Iranshahi, M. Biologically active prenylated flavonoids from the genus Sophora and their structure–activity relationship—A review. Phytother. Res. 2019, 33, 546–560. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, Q.; He, X.; Zhao, C.; Ren, W.; Yang, Z.; Wang, Z.; Ding, Q.; Deng, H.; Wang, T.; et al. Loss of Spike N370 glycosylation as an important evolutionary event for the enhanced infectivity of SARS-CoV-2. Cell Res. 2022, 32, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Tobita, K. Permanent canine kidney (MDCK) cells for isolation and plaque assay of influenza B viruses. Med. Microbiol. Immunol. 1975, 162, 23–27. [Google Scholar] [CrossRef]
- Alnajjar, R.; Mostafa, A.; Kandeil, A.; Al-Karmalawy, A.A. Molecular docking, molecular dynamics, and in vitro studies reveal the potential of angiotensin II receptor blockers to inhibit the COVID-19 main protease. Heliyon 2020, 6, e05641. [Google Scholar] [CrossRef]
- Elebeedy, D.; Badawy, I.; Elmaaty, A.A.; Saleh, M.M.; Kandeil, A.; Ghanem, A.; Kutkat, O.; Alnajjar, R.; Abd El Maksoud, A.I.; Al-Karmalawy, A.A. In vitro and computational insights revealing the potential inhibitory effect of Tanshinone IIA against influenza A virus. Comput. Biol. Med. 2022, 141, 105149. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Lin, L.C.; Tsai, W.J.; Chou, C.J.; Kung, S.H.; Ho, Y.H. Samarangenin B from Limonium sinense suppresses herpes simplex virus type 1 replication in Vero cells by regulation of viral macromolecular synthesis. Antimicrob. Agents Chemother. 2002, 46, 2854–2864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harcourt, J.; Tamin, A.; Lu, X.; Kamili, S.; Sakthivel, S.K.; Murray, J.; Queen, K.; Tao, Y.; Paden, C.R.; Zhang, J. Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease, United States. Emerg. Infect. Dis. 2020, 26, 1266. [Google Scholar] [CrossRef] [PubMed]
- Schuhmacher, A.; Reichling, J.; Schnitzler, P. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomed. Int. J. Phytother. Phytopharm. 2003, 10, 504–510. [Google Scholar] [CrossRef] [Green Version]
- Kandeil, A.; Mostafa, A.; Kutkat, O.; Moatasim, Y.; Al-Karmalawy, A.A.; Rashad, A.A.; Kayed, A.E.; Kayed, A.E.; El-Shesheny, R.; Kayali, G.; et al. Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2. Pathogens 2021, 10, 758. [Google Scholar] [CrossRef]
- Chemical Computing Group Inc. Molecular Operating Environment (MOE); Chemical Computing Group Inc.: Montreal, QC, Canada, 2016; Volume 1010. [Google Scholar]
- Mahmoud, D.B.; Bakr, M.M.; Al-Karmalawy, A.A.; Moatasim, Y.; El Taweel, A.; Mostafa, A. Scrutinizing the feasibility of nonionic surfactants to form isotropic bicelles of curcumin: A potential antiviral candidate against COVID-19. AAPS PharmSciTech 2022, 23, 1–12. [Google Scholar] [CrossRef]
- Elmaaty, A.A.; Darwish, K.M.; Chrouda, A.; Boseila, A.A.; Tantawy, M.A.; Elhady, S.S.; Shaik, A.B.; Mustafa, M.; Al-karmalawy, A.A. In Silico and In Vitro Studies for Benzimidazole Anthelmintics Repurposing as VEGFR-2 Antagonists: Novel Mebendazole-Loaded Mixed Micelles with Enhanced Dissolution and Anticancer Activity. ACS Omega 2022, 7, 875–899. [Google Scholar] [CrossRef]
- Hazem, R.M.; Antar, S.A.; Nafea, Y.K.; Al-Karmalawy, A.A.; Saleh, M.A.; El-Azab, M.F. Pirfenidone and vitamin D mitigate renal fibrosis induced by doxorubicin in mice with Ehrlich solid tumor. Life Sci. 2022, 288, 120185. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.I.A.; Darwish, K.M.; Soltane, R.; Chrouda, A.; Mostafa, A.; Abo Shama, N.M.; Elhady, S.S.; Abulkhair, H.S.; Khodir, A.E.; Elmaaty, A.A.; et al. β-Blockers bearing hydroxyethylamine and hydroxyethylene as potential SARS-CoV-2 Mpro inhibitors: Rational based design, in silico, in vitro, and SAR studies for lead optimization. RSC Adv. 2021, 11, 35536–35558. [Google Scholar] [CrossRef] [PubMed]
- Elia, S.G.; Al-Karmalawy, A.A.; Nasr, M.Y.; Elshal, M.F. Loperamide potentiates doxorubicin sensitivity in triple-negative breast cancer cells by targeting MDR1 and JNK and suppressing mTOR and Bcl-2: In vitro and molecular docking study. J. Biochem. Mol. Toxicol. 2022, 36, e22938. [Google Scholar] [CrossRef] [PubMed]
- Diab, R.T.; Abdel-Sami, Z.K.; Abdel-Aal, E.H.; Al-Karmalawy, A.A.; Abo-Dya, N.E. Design and synthesis of a new series of 3,5-disubstituted-1,2,4-oxadiazoles as potential colchicine binding site inhibitors: Antiproliferative activity, molecular docking, and SAR studies. New J. Chem. 2021, 45, 21657–21669. [Google Scholar] [CrossRef]
- Raslan, M.A.F.; Taher, R.; Al-Karmalawy, A.A.; El-Ebeedy, D.; Metwaly, A.G.; Elkateeb, N.M.; Ghanem, A.; Elghaish, R.A.; Abd El Maksoud,, A.I. Cordyline fruticosa (L.) A. Chev. leaves: Isolation, HPLC/MS profiling and evaluation of nephroprotective and hepatoprotective activities supported by molecular docking. New J. Chem. 2021, 45, 22216–22233. [Google Scholar] [CrossRef]
- Taher, R.F.; Al-Karmalawy, A.A.; Abd El Maksoud, A.I.; Khalil, H.; Hassan, A.; El-Khrisy, E.-D.A.; El-Kashak, W. Two new flavonoids and anticancer activity of Hymenosporum flavum: In vitro and molecular docking studies. J Herbmed Pharm. 2021, 10, 443–458. [Google Scholar] [CrossRef]
- Mahmoud, D.B.; Ismail, W.M.; Moatasim, Y.; Kutkat, O.; ElMeshad, A.N.; Ezzat, S.M.; El Deeb, K.S.; El-Fishawy, A.M.; Gomaa, M.R.; Kandeil, A.; et al. Delineating a potent antiviral activity of Cuphea ignea extract loaded nano-formulation against SARS-CoV-2: In silico and in vitro studies. J. Drug Deliv. Sci. Technol. 2021, 66, 102845. [Google Scholar] [CrossRef]
- Mansour, K.A.; Elbermawi, A.; Al-Karmalawy, A.A.; Lahloub, M.-F.; El-Neketi, M. Cytotoxic effects of extracts obtained from plants of the Oleaceae family: Bio-guided isolation and molecular docking of new secoiridoids from Jasminum humile. Pharm. Biol. 2022, 60, 1374–1383. [Google Scholar] [CrossRef]
- Salem, M.A.; El-Shiekh, R.A.; Aborehab, N.M.; Al-Karmalawy, A.A.; Ezzat, S.M.; Alseekh, S.; Fernie, A.R. Metabolomics driven analysis of Nigella sativa seeds identifies the impact of roasting on the chemical composition and immunomodulatory activity. Food Chem. 2022, 398, 133906. [Google Scholar] [CrossRef]
- El-Masry, R.M.; Al-Karmalawy, A.A.; Alnajjar, R.; Mahmoud, S.H.; Mostafa, A.; Kadry, H.H.; Abou-Seri, S.M.; Taher, A.T. Newly synthesized series of oxoindole–oxadiazole conjugates as potential anti-SARS-CoV-2 agents: In silico and in vitro studies. New J. Chem. 2022, 46, 5078–5090. [Google Scholar] [CrossRef]
- Aljuhani, A.; Ahmed, H.E.; Ihmaid, S.K.; Omar, A.M.; Althagfan, S.S.; Alahmadi, Y.M.; Ahmad, I.; Patel, H.; Ahmed, S.; Almikhlafi, M.A. In vitro and computational investigations of novel synthetic carboxamide-linked pyridopyrrolopyrimidines with potent activity as SARS-CoV-2-M Pro inhibitors. RSC Adv. 2022, 12, 26895–26907. [Google Scholar] [CrossRef] [PubMed]







| Name of Plant | Conc. (µg/mL) | Viral Count (PFU/mL) | Viral Count after Treatment (PFU/mL) | Inhibition % |
|---|---|---|---|---|
| Azadirachta indica (Neem) | 3.13 | 2.6 × 10−5 | 1.2 × 10−5 | 54% |
| 1.56 | 2.0 × 10−5 | 42% | ||
| 0.78 | 1.5 × 10−5 | 23% | ||
| Artemisia judaica (Shih-Balady) | 12.50 | 2.6 × 10−5 | 2 × 10−4 | 92% |
| 6.25 | 3 × 10−4 | 88% | ||
| 3.13 | 4 × 10−4 | 85% | ||
| Sophora tomentosa (Yellow Necklacepod) | 12.50 | 2.6 × 10−5 | 1 × 10−4 | 96% |
| 6.25 | 2 × 10−4 | 92% | ||
| 3.13 | 3 × 10−4 | 88% |
| Conc (µg/mL) | MERS-CoV | SARS-CoV-2 | ||||
|---|---|---|---|---|---|---|
| Viral Count (PFU/mL) | Viral Count after Treatment (PFU/mL) | Inhibition % | Viral Count (PFU/mL) | Viral Count after Treatment (PFU/mL) | Inhibition % | |
| 12.50 | 2.6 × 105 | 1 × 104 | 96% | 80 × 104 | 0 | 100% |
| 6.25 | 2 × 104 | 92% | 0 | 100% | ||
| 3.12 | 3 × 104 | 88% | 1 × 104 | 98.75% | ||
| No. | Comp. | Receptor | a S | RMSD | 2D Interaction | 3D Interaction | 3D Positioning |
|---|---|---|---|---|---|---|---|
| 4 | Genistein 4’-methyl ether | S | −5.71 | 1.77 |  |  |  |
| Mpr° | −6.44 | 0.80 |  |  |  | ||
| 8 | 6-Methoxy-7-O-β-D-glucoside apigenin | S | −7.03 | 2.13 |  |  |  |
| Mpr° | −7.36 | 1.12 |  |  |  | ||
| 10 | O6K | Mpr° | −8.98 | 1.99 |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Alla, H.I.; Kutkat, O.; Sweelam, H.-t.M.; Eldehna, W.M.; Mostafa, M.A.; Ibrahim, M.T.; Moatasim, Y.; GabAllah, M.; Al-Karmalawy, A.A. Investigating the Potential Anti-SARS-CoV-2 and Anti-MERS-CoV Activities of Yellow Necklacepod among Three Selected Medicinal Plants: Extraction, Isolation, Identification, In Vitro, Modes of Action, and Molecular Docking Studies. Metabolites 2022, 12, 1109. https://doi.org/10.3390/metabo12111109
Abd-Alla HI, Kutkat O, Sweelam H-tM, Eldehna WM, Mostafa MA, Ibrahim MT, Moatasim Y, GabAllah M, Al-Karmalawy AA. Investigating the Potential Anti-SARS-CoV-2 and Anti-MERS-CoV Activities of Yellow Necklacepod among Three Selected Medicinal Plants: Extraction, Isolation, Identification, In Vitro, Modes of Action, and Molecular Docking Studies. Metabolites. 2022; 12(11):1109. https://doi.org/10.3390/metabo12111109
Chicago/Turabian StyleAbd-Alla, Howaida I., Omnia Kutkat, Heba-tollah M. Sweelam, Wagdy M. Eldehna, Marwa A. Mostafa, Magda T. Ibrahim, Yassmin Moatasim, Mohamed GabAllah, and Ahmed A. Al-Karmalawy. 2022. "Investigating the Potential Anti-SARS-CoV-2 and Anti-MERS-CoV Activities of Yellow Necklacepod among Three Selected Medicinal Plants: Extraction, Isolation, Identification, In Vitro, Modes of Action, and Molecular Docking Studies" Metabolites 12, no. 11: 1109. https://doi.org/10.3390/metabo12111109