Enhancing Metabolic Imaging of Energy Metabolism in Traumatic Brain Injury Using Hyperpolarized [1-13C]Pyruvate and Dichloroacetate
Abstract
:1. Introduction
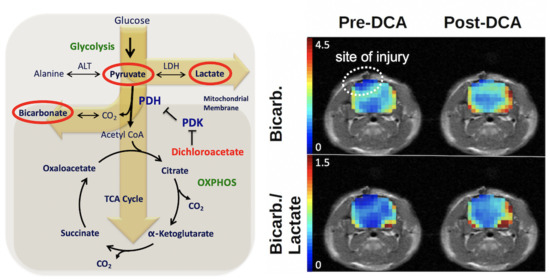
2. Results
3. Discussion
4. Materials and Methods
4.1. Controlled Cortical Impact (CCI)
4.2. Animal Handling during Magnetic Resonance Imaging (MRI)
4.3. Polarization and Injection
4.4. MR Acquisitions
4.5. Dichloroacetate
4.6. Longitudinal Study
4.7. Data Processing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roozenbeek, B.; Maas, A.I.R.; Menon, D.K. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 2013, 9, 231–236. [Google Scholar] [CrossRef]
- Coronado, V.G.; McGuire, L.C.; Faul, M.F.; Sugerman, D.E.; Pearson, W.S. Traumatic brain injury epidemiology and public health issues. In Brain Injury Medicine: Principles and Practice; Zasler, N.D., Katz, D.I., Zafonte, R.D., Eds.; Demos Medical: New York, NY, USA, 2012; pp. 84–100. [Google Scholar]
- Kolias, A.; Guilfoyle, M.R.; Helmy, A.; Allanson, J.; Hutchinson, P.J. Traumatic brain injury in adults. Pract. Neurol. 2013, 13, 228–235. [Google Scholar] [CrossRef] [Green Version]
- McKenna, M.C.; Scafidi, S.; Robertson, C.L. Metabolic Alterations in Developing Brain after Injury: Knowns and Unknowns. Neurochem. Res. 2015, 40, 2527–2543. [Google Scholar] [CrossRef] [Green Version]
- Jalloh, I.; Helmy, A.; Shannon, R.J.; Gallagher, C.N.; Menon, D.K.; Carpenter, K.L.H.; Hutchinson, P.J. Lactate uptake by the injured human brain: Evidence from an arteriovenous gradient and cerebral microdialysis study. J. Neurotrauma 2013, 30, 2031–2037. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, C.N.; Carpenter, K.L.H.; Grice, P.; Howe, D.J.; Mason, A.; Timofeev, I.; Menon, D.K.; Kirkpatrick, P.J.; Pickard, J.D.; Sutherland, G.R.; et al. The human brain utilizes lactate via the tricarboxylic acid cycle: A 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain 2009, 132, 2839–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalloh, I.; Carpenter, K.L.H.; Helmy, A.; Carpenter, T.A.; Menon, D.K.; Hutchinson, P.J. Glucose metabolism following human traumatic brain injury: Methods of assessment and pathophysiological findings. Metab. Brain. Dis. 2015, 30, 615–632. [Google Scholar] [CrossRef] [Green Version]
- Byrnes, K.R.; Wilson, C.M.; Brabazon, F.; von Leden, R.; Jurgens, J.S.; Oakes, T.R.; Selwyn, R.G. FDG-PET imaging in mild traumatic brain injury: A critical review. Front. Neuroenerg. 2014, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- DeVience, S.J.; Lu, X.; Proctor, J.; Rangghran, P.; Melhelm, E.R.; Gullapalli, R.; Fiskum, G.M.; Mayer, D. Metabolic imaging of energy metabolism in traumatic brain injury using hyperpolarized [1-13C]pyruvate. Sci. Rep. 2017, 7, 1907. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, C.; Chou, A.; Krukowski, K.; Najac, C.; Feng, X.; Riparip, L.-K.; Rosi, S.; Chaumeil, M.M. In vivo metabolic imaging of traumatic brain injury. Sci. Rep. 2017, 7, 17525. [Google Scholar] [CrossRef] [Green Version]
- Stoica, B.A.; Faden, A.I. Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics 2010, 7, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Benford, B.; Li, Z.Z.; Ling, G.S.F. Role of pyruvate dehydrogenase complex in traumatic brain injury and measurement of pyruvate dehydrogenase enzyme by dipstick test. J. Emerg. Trauma Shock 2009, 2, 67–72. [Google Scholar] [CrossRef]
- Xing, G.; Ren, M.; Watson, W.A.; O’Neil, J.T.; Verma, A. Traumatic brain injury-induced expression and phosphorylation of pyruvate dehydrogenase: A mechanism of dysregulated glucose metabolism. Neurosci. Lett. 2009, 454, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Recht, L.; Josan, S.; Merchant, M.; Jang, T.; Yen, Y.-F.; Hurd, R.; Spielman, D.; Mayer, D. Metabolic Response of Glioma to Dichloroacetate Measured in vivo by Hyperpolarized 13C Magnetic Resonance Spectroscopic Imaging. Neuro-Oncology 2013, 15, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Long, J.A.; Watts, L.T.; Chemello, J.; Huang, S.; Shen, Q.; Duong, T.Q. Multiparametric and Longitudinal MRI Characterization of Mild Traumatic Brain Injury in Rats. J. Neurotrauma 2015, 32, 598–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.-L.; Yu, M.-M.; Yang, D.-X.; Liu, Y.-L.; Wei, X.-E.; Li, W.-B. Longitudinal Microstructural Changes in Traumatic Brain Injury in Rats: A Diffusional Kurtosis Imaging, Histology, and Behavior Study. Am. J. Neuroradiol. 2018, 39, 1650–1656. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Liu, X.; Kida, K.; Marutani, E.; Hirai, S.; Sakaguchi, M.; Andersen, L.W.; Bagchi, A.; Cocchi, M.N.; Berg, K.M.; et al. Thiamine as a Neuroprotective Agent after Cardiac Arrest. Resuscitation 2016, 105, 138–144. [Google Scholar] [CrossRef]
- Holness, M.J.; Sugden, M.C. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc. Trans. 2003, 31, 1143–1151. [Google Scholar] [CrossRef]
- Randle, P.J.; Denton, R.M. Regulation of Pyruvate Dehydrogenase by End Product Inhibition and by Phosphorylation. In Metabolic Interconversion of Enzymes 1975. Proceedings in Life Sciences; Shaltiel, S., Ed.; Springer: Berlin, Germany, 1976; pp. 136–141. [Google Scholar] [CrossRef]
- Bogaert, Y.E.; Rosenthal, R.E.; Fiskum, G. Postischemic Inhibition of Cerebral Cortex Pyruvate Dehydrogenase. Free Radic Biol. Med. 1994, 16, 811–820. [Google Scholar] [CrossRef]
- Robertson, C.L.; Saraswati, M.; Fiskum, G. Mitochondrial dysfunction early after traumatic brain injury in immature rats. J. Neurochem. 2007, 101, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, T.; Xie, C.; Zhang, Y.; Zhou, K.; Wang, X.; Blomgren, K.; Zhu, C. Dichloroacetate treatment improves mitochondrial metabolism and reduces brain injury in neonatal mice. Oncotarget 2016, 7, 1708–1722. [Google Scholar] [CrossRef] [Green Version]
- Sutendra, G.; Dromparis, P.; Kinnaird, A.; Stenson, T.H.; Haromy, A.; Parker, J.M.R.; McMurtry, M.S.; Michelakis, E.D. Mitochondrial activation by inhibition of PDKII suppresses HIF1a signaling and angiogenesis in cancer. Oncogene 2013, 32, 1638–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arambula, S.E.; Reinl, E.L.; El Demerdash, N.; McCarthy, M.M.; Robertson, C.L. Sex Differences in Pediatric Traumatic Brain Injury. Exp. Neurol. 2019, 317, 168–179. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: New York, NY, USA, 1998. [Google Scholar]
- Xu, S.; Zhuo, J.; Racz, J.; Shi, D.; Roys, S.; Fiskum, G.; Gullapalli, R. Early microstructural and metabolic changes following controlled cortical impact injury in rat: A magnetic resonance imaging and spectroscopy study. J. Neurotrauma 2011, 28, 2091–2102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurd, R.E.; Yen, Y.-F.; Mayer, D.; Chen, A.; Wilson, D.; Kohler, S.; Bok, R.; Vigneron, D.; Kurhanewicz, J.; Tropp, J.; et al. Metabolic imaging in the anesthetized rat brain using hyperpolarized [1-13C] pyruvate and [1-13C] ethyl pyruvate. Magn. Reson. Med. 2010, 63, 1137–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, D.; Yen, Y.-F.; Levin, Y.S.; Tropp, J.; Pfefferbaum, A.; Hurd, R.E.; Spielman, D.M. In Vivo Application of Sub-Second Spiral Chemical Shift Imaging (CSI) to Hyperpolarized 13C Metabolic Imaging: Comparison with Phase-Encoded CSI. J. Magn. Reson. 2010, 204, 340–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Group | Day 0 | Day 2 | Day 7 | Day 28 | ||||
|---|---|---|---|---|---|---|---|---|
| DCA | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Control | 11 | 4 | - | - | - | - | - | - |
| Sham | 4 | 3 | 4 | 4 | 4 | 4 | 0 | 0 |
| CCI | 6 | 3 | 4 | 4 | 4 | 2 | 4 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeVience, S.J.; Lu, X.; Proctor, J.L.; Rangghran, P.; Medina, J.A.; Melhem, E.R.; Gullapalli, R.P.; Fiskum, G.; Mayer, D. Enhancing Metabolic Imaging of Energy Metabolism in Traumatic Brain Injury Using Hyperpolarized [1-13C]Pyruvate and Dichloroacetate. Metabolites 2021, 11, 335. https://doi.org/10.3390/metabo11060335
DeVience SJ, Lu X, Proctor JL, Rangghran P, Medina JA, Melhem ER, Gullapalli RP, Fiskum G, Mayer D. Enhancing Metabolic Imaging of Energy Metabolism in Traumatic Brain Injury Using Hyperpolarized [1-13C]Pyruvate and Dichloroacetate. Metabolites. 2021; 11(6):335. https://doi.org/10.3390/metabo11060335
Chicago/Turabian StyleDeVience, Stephen J., Xin Lu, Julie L. Proctor, Parisa Rangghran, Juliana A. Medina, Elias R. Melhem, Rao P. Gullapalli, Gary Fiskum, and Dirk Mayer. 2021. "Enhancing Metabolic Imaging of Energy Metabolism in Traumatic Brain Injury Using Hyperpolarized [1-13C]Pyruvate and Dichloroacetate" Metabolites 11, no. 6: 335. https://doi.org/10.3390/metabo11060335