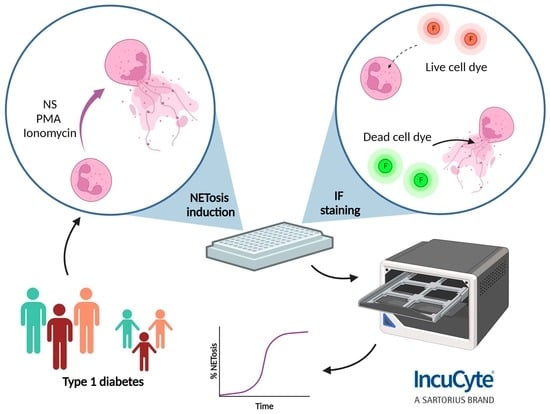
High-Throughput Analysis of Neutrophil Extracellular Trap Levels in Subtypes of People with Type 1 Diabetes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects and Ethics Statement
2.2. Isolation of Primary Human Neutrophils
2.3. Measurements of NET Markers
2.4. NET Induction and Staining for Incucyte® ZOOM
2.5. Imaging and Quantification of NETs with Incucyte® ZOOM
2.6. Visualization of NETs by Confocal Microscopy
2.7. Bioinformatics and Statistical Analyses
3. Results
3.1. Identification of NET Formation in Response to Distinct Stimuli by Incucyte® ZOOM
3.2. Using Incucyte® ZOOM to Determine Levels of NET Formation in Subtypes of Adults and Children with Type 1 Diabetes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wigerblad, G.; Kaplan, M.J. Neutrophil Extracellular Traps in Systemic Autoimmune and Autoinflammatory Diseases. Nat. Rev. Immunol. 2022, 23, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Bissenova, S.; Ellis, D.; Mathieu, C.; Gysemans, C. Neutrophils in Autoimmunity: When the Hero Becomes the Villain. Clin. Exp. Immunol. 2022, 210, 128–140. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Ogle, G.D.; Lund-Blix, N.A.; Stene, L.C. Update on Worldwide Trends in Occurrence of Childhood Type 1 Diabetes in 2020. Pediatr. Endocrinol. Rev. PER 2020, 17, 198–209. [Google Scholar] [CrossRef]
- Peters, L.; Posgai, A.; Brusko, T.M. Islet–Immune Interactions in Type 1 Diabetes: The Nexus of Beta Cell Destruction. Clin. Exp. Immunol. 2019, 198, 326–340. [Google Scholar] [CrossRef] [Green Version]
- Vecchio, F.; Buono, N.L.; Stabilini, A.; Nigi, L.; Dufort, M.J.; Geyer, S.; Rancoita, P.M.; Cugnata, F.; Mandelli, A.; Valle, A.; et al. Abnormal Neutrophil Signature in the Blood and Pancreas of Presymptomatic and Symptomatic Type 1 Diabetes. JCI Insight 2018, 3, e122146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolaczkowska, E.; Kubes, P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Dragunow, M.; Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Requirements for NADPH Oxidase and Myeloperoxidase in Neutrophil Extracellular Trap Formation Differ Depending on the Stimulus. J. Leukoc. Biol. 2012, 92, 841–849. [Google Scholar] [CrossRef]
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; von Bernuth, H.; Zychlinsky, A. Diverse Stimuli Engage Different Neutrophil Extracellular Trap Pathways. eLife 2017, 6, e24437. [Google Scholar] [CrossRef] [PubMed]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 Channel and Mitochondrial ROS Mediate NADPH Oxidase-Independent NETosis Induced by Calcium Influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Fresneda Alarcon, M.; McLaren, Z.; Wright, H.L. Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O. Front. Immunol. 2021, 12, 649693. [Google Scholar] [CrossRef]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef] [Green Version]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; et al. Neutrophils Activate Plasmacytoid Dendritic Cells by Releasing Self-DNA–Peptide Complexes in Systemic Lupus Erythematosus. Sci. Transl. Med. 2011, 3, 73ra19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Romo, G.S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; et al. Netting Neutrophils Are Major Inducers of Type I IFN Production in Pediatric Systemic Lupus Erythematosus. Sci. Transl. Med. 2011, 3, 73ra20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Söderberg, D.; Segelmark, M. Neutrophil Extracellular Traps in ANCA-Associated Vasculitis. Front. Immunol. 2016, 7, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.-J.; Brinkmann, V.; Jenne, D.E. Netting Neutrophils in Autoimmune Small-Vessel Vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Zhong, L.; Ye, D.; Zhang, J.; Tu, Y.; Bornstein, S.R.; Zhou, Z.; Lam, K.S.L.; Xu, A. Increased Neutrophil Elastase and Proteinase 3 and Augmented NETosis Are Closely Associated With β-Cell Autoimmunity in Patients With Type 1 Diabetes. Diabetes 2014, 63, 4239–4248. [Google Scholar] [CrossRef] [Green Version]
- Klocperk, A.; Vcelakova, J.; Vrabcova, P.; Zentsova, I.; Petruzelkova, L.; Sumnik, Z.; Pruhova, S.; Sediva, A.; Parackova, Z. Elevated Biomarkers of NETosis in the Serum of Pediatric Patients With Type 1 Diabetes and Their First-Degree Relatives. Front. Immunol. 2021, 12, 699386. [Google Scholar] [CrossRef] [PubMed]
- Skoglund, C.; Appelgren, D.; Johansson, I.; Casas, R.; Ludvigsson, J. Increase of Neutrophil Extracellular Traps, Mitochondrial DNA and Nuclear DNA in Newly Diagnosed Type 1 Diabetes Children but Not in High-Risk Children. Front. Immunol. 2021, 12, 628564. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Fu, S.; Speake, C.; Greenbaum, C.J.; Odegard, J.M. NETosis-Associated Serum Biomarkers Are Reduced in Type 1 Diabetes in Association with Neutrophil Count. Clin. Exp. Immunol. 2016, 184, 318–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aukrust, S.G.; Holte, K.B.; Opstad, T.B.; Seljeflot, I.; Berg, T.J.; Helseth, R. NETosis in Long-Term Type 1 Diabetes Mellitus and Its Link to Coronary Artery Disease. Front. Immunol. 2021, 12, 799539. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Ahmed, S.; Anderson, M.S.; Atkinson, M.A.; Becker, D.; Bingley, P.J.; Bosi, E.; Brusko, T.M.; DiMeglio, L.A.; Evans-Molina, C.; et al. Introducing the Endotype Concept to Address the Challenge of Disease Heterogeneity in Type 1 Diabetes. Diabetes Care 2020, 43, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Insel, R.A.; Dunne, J.L.; Atkinson, M.A.; Chiang, J.L.; Dabelea, D.; Gottlieb, P.A.; Greenbaum, C.J.; Herold, K.C.; Krischer, J.P.; Lernmark, Å.; et al. Staging Presymptomatic Type 1 Diabetes: A Scientific Statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015, 38, 1964–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, L.G.; Ostuni, R.; Hidalgo, A. Heterogeneity of Neutrophils. Nat. Rev. Immunol. 2019, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Bissenova, S.; Ellis, D.; Callebaut, A.; Eelen, G.; Derua, R.; Buitinga, M.; Mathieu, C.; Gysemans, C.; Overbergh, L. NET Proteome in Established Type 1 Diabetes Is Enriched in Metabolic Proteins. Cells 2023, 12, 1319. [Google Scholar] [CrossRef]
- Gupta, S.; Chan, D.W.; Zaal, K.J.; Kaplan, M.J. A High Throughput Real-Time Imaging Technique to Quantify NETosis and Distinguish Mechanisms of Cell Death in Human Neutrophils. J. Immunol. 2018, 200, 869–879. [Google Scholar] [CrossRef] [Green Version]
- Parker, H.A.; Jones, H.M.; Kaldor, C.D.; Hampton, M.B.; Winterbourn, C.C. Neutrophil NET Formation with Microbial Stimuli Requires Late Stage NADPH Oxidase Activity. Antioxidants 2021, 10, 1791. [Google Scholar] [CrossRef]
- Huang, J.; Hong, W.; Wan, M.; Zheng, L. Molecular Mechanisms and Therapeutic Target of NETosis in Diseases. MedComm 2022, 3, e162. [Google Scholar] [CrossRef]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable Neutrophils Release Mitochondrial DNA to Form Neutrophil Extracellular Traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Cristinziano, L.; Modestino, L.; Loffredo, S.; Varricchi, G.; Braile, M.; Ferrara, A.L.; de Paulis, A.; Antonelli, A.; Marone, G.; Galdiero, M.R. Anaplastic Thyroid Cancer Cells Induce the Release of Mitochondrial Extracellular DNA Traps by Viable Neutrophils. J. Immunol. 2020, 204, 1362–1372. [Google Scholar] [CrossRef]
- McIlroy, D.J.; Jarnicki, A.G.; Au, G.G.; Lott, N.; Smith, D.W.; Hansbro, P.M.; Balogh, Z.J. Mitochondrial DNA Neutrophil Extracellular Traps Are Formed after Trauma and Subsequent Surgery. J. Crit. Care 2014, 29, 1133.e1–1133.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Aziz, M.; Wang, P. The Vitals of NETs. J. Leukoc. Biol. 2021, 110, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Acebes, M.; Pitaval, C.; Weiss, L.A.; Nombela-Arrieta, C.; Chèvre, R.; A-González, N.; Kunisaki, Y.; Zhang, D.; van Rooijen, N.; Silberstein, L.E.; et al. Rhythmic Modulation of the Hematopoietic Niche through Neutrophil Clearance. Cell 2013, 153, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Hayden, H.; Ibrahim, N.; Klopf, J.; Zagrapan, B.; Mauracher, L.-M.; Hell, L.; Hofbauer, T.M.; Ondracek, A.S.; Schoergenhofer, C.; Jilma, B.; et al. ELISA Detection of MPO-DNA Complexes in Human Plasma Is Error-Prone and Yields Limited Information on Neutrophil Extracellular Traps Formed In Vivo. PLoS ONE 2021, 16, e0250265. [Google Scholar] [CrossRef]
- Tate, J.; Ward, G. Interferences in Immunoassay. Clin. Biochem. Rev. 2004, 25, 105–120. [Google Scholar] [PubMed]
- Boeltz, S.; Amini, P.; Anders, H.-J.; Andrade, F.; Bilyy, R.; Chatfield, S.; Cichon, I.; Clancy, D.M.; Desai, J.; Dumych, T.; et al. To NET or Not to NET:Current Opinions and State of the Science Regarding the Formation of Neutrophil Extracellular Traps. Cell Death Differ. 2019, 26, 395–408. [Google Scholar] [CrossRef] [Green Version]
- Gierlikowska, B.; Stachura, A.; Gierlikowski, W.; Demkow, U. Phagocytosis, Degranulation and Extracellular Traps Release by Neutrophils—The Current Knowledge, Pharmacological Modulation and Future Prospects. Front. Pharmacol. 2021, 12, 666732. [Google Scholar] [CrossRef]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes Primes Neutrophils to Undergo NETosis, Which Impairs Wound Healing. Nat. Med. 2015, 21, 815. [Google Scholar] [CrossRef] [Green Version]




| At Risk T1D | New Onset T1D | Established T1D | Healthy Control | |
|---|---|---|---|---|
| Number of individuals | 8 | 7 | 14 | 27 |
| Age (years) 1 | 26 (21–33) | 29 (22–42) | 30 (20–45) | 28 (22–45) |
| Disease duration (years) 1 | NA | NA | 11 (1–28) | NA |
| Disease duration (days) 1 | NA | 28 (13–49) | NA | NA |
| Age at diagnosis (years) 1 | NA | 29 (22–42) | 19 (4–32) | NA |
| Gender F/M | 4/4 | 5/2 | 6/8 | 17/10 |
| Glycemia (mg/dL) 1 | NA | 105.4 (89–137) | 176 (55–320) | NA |
| HbA1c (%) 1 | NA | 7.5 (6.5–10) | 7.1 (5.0–9.2) | NA |
| TIR (%) 1,2 | NA | NA | 70 (37–90) | NA |
| Insulin dosage (U/day) | NA | 24 (4–56) | 49.0 (12–73) | NA |
| GADA (U/mL) pos/neg 3 | 4/4 | 6/1 | 8/6 | 0/27 |
| IA2-A (U/mL) pos/neg 4 | 1/7 | 3/4 | 7/7 | 0/27 |
| IAA (% binding) pos/neg 5 | 2/6 | 2/5 | 8/6 | 0/27 |
| ZnT8A (% binding) pos/neg 6 | 1/7 | 0/7 | 1/13 | 0/27 |
| At Risk T1D | New Onset T1D | Established T1D | Healthy Control | |
|---|---|---|---|---|
| Number of individuals | 6 | 5 | 6 | 11 |
| Age (years) 1 | 10 (8–14) | 12 (6–16) | 14 (9–17) | 8 (5–13) |
| Disease duration (years) 1 | NA | NA | 7 (3–15) | NA |
| Disease duration (days) 1 | NA | 20 (7–42) | NA | NA |
| Age at diagnosis (years) 1 | NA | 12 (6–16) | 8 (1–12) | NA |
| Gender F/M | 3/3 | 3/2 | 3/3 | 5/6 |
| Glycemia (mg/dL) 1 | 100 (66–125) | 287 (90–740) | 403.5 (213–502) | NA |
| HbA1c (%) 1 | 5.5 (5.3–5.7) | 9.8 (7.3–13.4) | 6.7 (6.5–7) | NA |
| TIR (%) 1,2 | NA | NA | 70.5 (69–72) | NA |
| Insulin dosage (U/day) | NA | 32.2 (12–66) | 48.8 (40–58) | NA |
| GADA (U/mL) pos/neg 3 | 5/1 | 3/2 | 3/3 | 0/11 |
| IA2-A (U/mL) pos/neg 4 | 4/2 | 4/1 | 2/4 | 0/11 |
| IAA (% binding) pos/neg 5 | 1/5 | 3/2 | 4/2 | 0/11 |
| ZnT8A (% binding) pos/neg 6 | 1/5 | 3/2 | 1/5 | 0/11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bissenova, S.; Buitinga, M.; Boesch, M.; Korf, H.; Casteels, K.; Teunkens, A.; Mathieu, C.; Gysemans, C. High-Throughput Analysis of Neutrophil Extracellular Trap Levels in Subtypes of People with Type 1 Diabetes. Biology 2023, 12, 882. https://doi.org/10.3390/biology12060882
Bissenova S, Buitinga M, Boesch M, Korf H, Casteels K, Teunkens A, Mathieu C, Gysemans C. High-Throughput Analysis of Neutrophil Extracellular Trap Levels in Subtypes of People with Type 1 Diabetes. Biology. 2023; 12(6):882. https://doi.org/10.3390/biology12060882
Chicago/Turabian StyleBissenova, Samal, Mijke Buitinga, Markus Boesch, Hannelie Korf, Kristina Casteels, An Teunkens, Chantal Mathieu, and Conny Gysemans. 2023. "High-Throughput Analysis of Neutrophil Extracellular Trap Levels in Subtypes of People with Type 1 Diabetes" Biology 12, no. 6: 882. https://doi.org/10.3390/biology12060882