ALM Therapy Promotes Functional and Histologic Regeneration of Traumatized Peripheral Skeletal Muscle
Abstract
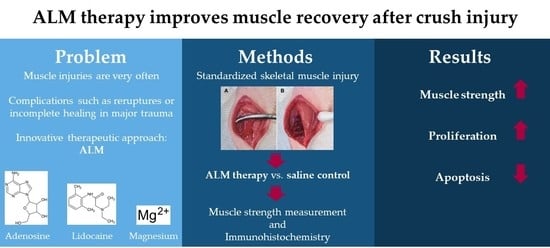
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model and Experimental Groups
2.2. Muscle Strength Measurement
2.3. Histochemistry and Immunohistochemistry
2.4. Statistical Analysis
3. Results
3.1. General Observations In Vivo
3.2. Muscle Strength Measurement
3.3. Muscle Tissue Proliferation and Apoptosis
4. Discussion
4.1. Animal Skeletal Muscle–Trauma Model
4.2. Groups and Post-Traumatic Examination Times
4.3. Timing, Dosage and Mode of Application of ALM
4.4. Muscle Strength Measurement
4.5. Muscle Cell Proliferation and Apoptosis after Crush Injury and ALM
4.6. Limitations of the Present Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kääriäinen, M.; Järvinen, T.; Järvinen, M.; Rantanen, J.; Kalimo, H. Relation between myofibers and connective tissue during muscle injury repair. Scand. J. Med. Sci. Sport. 2000, 10, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Rahusen, F.T.G.; Weinhold, P.S.; Almekinders, L.C. Nonsteroidal anti-inflammatory drugs and acetaminophen in the treatment of an acute muscle injury. Am. J. Sport. Med. 2004, 32, 1856–1859. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.; Gottfried, C. Muscle injury: Review of experimental models. J. Electromyogr. Kinesiol. 2013, 23, 1253–1260. [Google Scholar] [CrossRef]
- Prisk, V.; Huard, J. Muscle injuries and repair: The role of prostaglandins and inflammation. Histol. Histopathol. 2003, 18, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.A.; Järvinen, M.; Kalimo, H. Regeneration of injured skeletal muscle after the injury. Muscles Ligaments Tendons J. 2013, 3, 337–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huard, J.; Li, Y.; Fu, F.H. Muscle injuries and repair: Current trends in research. J. Bone Jt. Surg. Am. 2002, 84, 822–832. [Google Scholar] [CrossRef]
- Kujala, U.M.; Orava, S.; Järvinen, M. Hamstring injuries. Current trends in treatment and prevention. Sport. Med. 1997, 23, 397–404. [Google Scholar] [CrossRef]
- Menetrey, J.; Kasemkijwattana, C.; Fu, F.H.; Moreland, M.S.; Huard, J. Suturing versus immobilization of a muscle laceration. A morphological and functional study in a mouse model. Am. J. Sport. Med. 1999, 27, 222–229. [Google Scholar] [CrossRef]
- Aärimaa, V.; Kääriäinen, M.; Vaittinen, S.; Tanner, J.; Järvinen, T.; Best, T.; Kalimo, H. Restoration of myofiber continuity after transection injury in the rat soleus. Neuromuscul. Disord. 2004, 14, 421–428. [Google Scholar] [CrossRef]
- Ahmad, C.S.; Redler, L.H.; Ciccotti, M.G.; Maffulli, N.; Longo, U.G.; Bradley, J. Evaluation and management of hamstring injuries. Am. J. Sport. Med. 2013, 41, 2933–2947. [Google Scholar] [CrossRef]
- Hurme, T.; Kalimo, H.; Lehto, M.; Järvinen, M. Healing of skeletal muscle injury: An ultrastructural and immunohistochemical study. Med. Sci. Sport. Exerc. 1991, 23, 801–810. [Google Scholar] [CrossRef]
- Järvinen, T.A.H.; Järvinen, T.L.N.; Kääriäinen, M.; Kalimo, H.; Järvinen, M. Muscle injuries: Biology and treatment. Am. J. Sport. Med. 2005, 33, 745–764. [Google Scholar] [CrossRef]
- Tidball, J.G. Inflammatory cell response to acute muscle injury. Med. Sci. Sport. Exerc. 1995, 27, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.G.; St Pierre, B.A. Cytokines in exertion-induced skeletal muscle injury. Mol. Cell. Biochem. 1998, 179, 159–167. [Google Scholar] [CrossRef]
- Hurme, T.; Kalimo, H. Activation of myogenic precursor cells after muscle injury. Med. Sci. Sport. Exerc. 1992, 24, 197–205. [Google Scholar] [CrossRef]
- Järvinen, M. Healing of a crush injury in rat striated muscle. 3. A micro-angiographical study of the effect of early mobilization and immobilization on capillary ingrowth. Acta Pathol. Microbiol. Scand. A 1976, 84, 85–94. [Google Scholar] [PubMed]
- Lehto, M.; Duance, V.C.; Restall, D. Collagen and fibronectin in a healing skeletal muscle injury. An immunohistological study of the effects of physical activity on the repair of injured gastrocnemius muscle in the rat. J. Bone Jt. Surg. Br. 1985, 67, 820–828. [Google Scholar] [CrossRef] [Green Version]
- Lehto, M.; Sims, T.J.; Bailey, A.J. Skeletal muscle injury--molecular changes in the collagen during healing. Res. Exp. Med. 1985, 185, 95–106. [Google Scholar] [CrossRef]
- Kääriäinen, M.; Kääriäinen, J.; Järvinen, T.L.; Sievänen, H.; Kalimo, H.; Järvinen, M. Correlation between biomechanical and structural changes during the regeneration of skeletal muscle after laceration injury. J. Orthop. Res. 1998, 16, 197–206. [Google Scholar] [CrossRef]
- Vaittinen, S.; Hurme, T.; Rantanen, J.; Kalimo, H. Transected myofibres may remain permanently divided in two parts. Neuromuscul. Disord. 2002, 12, 584–587. [Google Scholar] [CrossRef]
- Dobson, G.P.; Letson, H.L. Adenosine, lidocaine, and Mg2+ (ALM): From cardiac surgery to combat casualty care--Teaching old drugs new tricks. J. Trauma Acute Care Surg. 2016, 80, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt, A.; Nielsen, T.K.; Sølling, C.; Hyldebrandt, J.A.; Frøkiær, J.; Wogensen, L.; Dobson, G.P.; Vinten-Johansen, J.; Tønnesen, E. Adenocaine and Mg2+ reduce fluid requirement to maintain hypotensive resuscitation and improve cardiac and renal function in a porcine model of severe hemorrhagic shock*. Crit. Care Med. 2012, 40, 3013–3025. [Google Scholar] [CrossRef]
- Granfeldt, A.; Letson, H.L.; Dobson, G.P.; Shi, W.; Vinten-Johansen, J.; Tønnesen, E. Adenosine, lidocaine and Mg2+ improves cardiac and pulmonary function, induces reversible hypotension and exerts anti-inflammatory effects in an endotoxemic porcine model. Crit. Care 2014, 18, 682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letson, H.L.; Dobson, G.P. 3% NaCl adenosine, lidocaine, Mg2+ (ALM) bolus and 4 hours “drip” infusion reduces noncompressible hemorrhage by 60% in a rat model. J. Trauma Acute Care Surg. 2017, 82, 1063–1072. [Google Scholar] [CrossRef]
- Letson, H.L.; Granfeldt, A.; Jensen, T.H.; Mattson, T.H.; Dobson, G.P. Adenosine, Lidocaine, and Magnesium Support a High Flow, Hypotensive, Vasodilatory State with Improved Oxygen Delivery and Cerebral Protection in a Pig Model of Noncompressible Hemorrhage. J. Surg. Res. 2020, 253, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.L.; Letson, H.L.; McEwen, P.; Biros, E.; Dlaska, C.; Hazratwala, K.; Wilkinson, M.; Dobson, G.P. Comparison of intra-articular administration of adenosine, lidocaine and magnesium solution and tranexamic acid for alleviating postoperative inflammation and joint fibrosis in an experimental model of knee arthroplasty. J. Orthop. Surg. Res. 2021, 16, 726. [Google Scholar] [CrossRef]
- Letson, H.L.; Dobson, G.P. Correction of acute traumatic coagulopathy with small-volume 7.5% NaCl adenosine, lidocaine, and Mg2+ occurs within 5 minutes: A ROTEM analysis. J. Trauma Acute Care Surg. 2015, 78, 773–783. [Google Scholar] [CrossRef]
- Letson, H.; Dobson, G. Adenosine, lidocaine and Mg2+ (ALM) fluid therapy attenuates systemic inflammation, platelet dysfunction and coagulopathy after non-compressible truncal hemorrhage. PLoS ONE 2017, 12, e0188144. [Google Scholar] [CrossRef] [Green Version]
- Letson, H.L.; Morris, J.L.; Biros, E.; Dobson, G.P. ALM Induces Cellular Quiescence in the Surgical Margin 3 Days Following Liver Resection, Hemorrhage, and Shock. J. Surg. Res. 2022, 275, 16–28. [Google Scholar] [CrossRef]
- Matziolis, G.; Winkler, T.; Schaser, K.; Wiemann, M.; Krocker, D.; Tuischer, J.; Perka, C.; Duda, G.N. Autologous bone marrow-derived cells enhance muscle strength following skeletal muscle crush injury in rats. Tissue Eng. 2006, 12, 361–367. [Google Scholar] [CrossRef]
- Ferreira, R.; Neuparth, M.J.; Ascensão, A.; Magalhães, J.; Vitorino, R.; Duarte, J.A.; Amado, F. Skeletal muscle atrophy increases cell proliferation in mice gastrocnemius during the first week of hindlimb suspension. Eur. J. Appl. Physiol. 2006, 97, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Rotter, R.; Menshykova, M.; Winkler, T.; Matziolis, G.; Stratos, I.; Schoen, M.; Bittorf, T.; Mittlmeier, T.; Vollmar, B. Erythropoietin improves functional and histological recovery of traumatized skeletal muscle tissue. J. Orthop. Res. 2008, 26, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Stratos, I.; Graff, J.; Rotter, R.; Mittlmeier, T.; Vollmar, B. Open blunt crush injury of different severity determines nature and extent of local tissue regeneration and repair. J. Orthop. Res. 2010, 28, 950–957. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Phys. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Gerdes, J.; Li, L.; Schlueter, C.; Duchrow, M.; Wohlenberg, C.; Gerlach, C.; Stahmer, I.; Kloth, S.; Brandt, E.; Flad, H.D. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am. J. Pathol. 1991, 138, 867–873. [Google Scholar]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.H.; Schwab, U.; Stein, H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar] [CrossRef]
- Gerdes, J.; Schwab, U.; Lemke, H.; Stein, H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer 1983, 31, 13–20. [Google Scholar] [CrossRef]
- Fedrowitz, M.; Westermann, J.; Löscher, W. Magnetic field exposure increases cell proliferation but does not affect melatonin levels in the mammary gland of female Sprague Dawley rats. Cancer Res. 2002, 62, 1356–1363. [Google Scholar]
- Enami, Y.; Kato, H.; Murakami, M.; Fujioka, T.; Aoki, T.; Niiya, T.; Murai, N.; Ohtsuka, K.; Kusano, M. Anti-transforming growth factor-beta1 antibody transiently enhances DNA synthesis during liver regeneration after partial hepatectomy in rats. J. Hepatobiliary Pancreat. Surg. 2001, 8, 250–258. [Google Scholar] [CrossRef]
- Birner, P.; Ritzi, M.; Musahl, C.; Knippers, R.; Gerdes, J.; Voigtländer, T.; Budka, H.; Hainfellner, J.A. Immunohistochemical detection of cell growth fraction in formalin-fixed and paraffin-embedded murine tissue. Am. J. Pathol. 2001, 158, 1991–1996. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Mitui, H.; Udaka, N.; Hayashi, H.; Okudela, K.; Kanisawa, M.; Kitamura, H. Ki-67 (MIB 5) immunostaining of mouse lung tumors induced by 4-nitroquinoline 1-oxide. Histochem. Cell Biol. 1998, 110, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Muskhelishvili, L.; Latendresse, J.R.; Kodell, R.L.; Henderson, E.B. Evaluation of cell proliferation in rat tissues with BrdU, PCNA, Ki-67(MIB-5) immunohistochemistry and in situ hybridization for histone mRNA. J. Histochem. Cytochem. 2003, 51, 1681–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobson, G.P.; Letson, H.L.; Sharma, R.; Sheppard, F.R.; Cap, A.P. Mechanisms of early trauma-induced coagulopathy: The clot thickens or not? J. Trauma Acute Care Surg. 2015, 79, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Griffin, M.J.; Letson, H.L.; Dobson, G.P. Small-Volume Adenosine, Lidocaine, and Mg2+ 4-Hour Infusion Leads to 88% Survival after 6 Days of Experimental Sepsis in the Rat without Antibiotics. Clin. Vaccine Immunol. 2016, 23, 863–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letson, H.L.; Morris, J.L.; Biros, E.; Dobson, G.P. Adenosine, lidocaine, and Mg2+ fluid therapy leads to 72-hour survival after hemorrhagic shock: A model for studying differential gene expression and extending biological time. J. Trauma Acute Care Surg. 2019, 87, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; von Roth, P.; Matziolis, G.; Schumann, M.R.; Hahn, S.; Strube, P.; Stoltenburg-Didinger, G.; Perka, C.; Duda, G.N.; Tohtz, S.V. Time course of skeletal muscle regeneration after severe trauma. Acta Orthop. 2011, 82, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Järvinen, M.; Sorvari, T. Healing of a crush injury in rat striated muscle. 1. Description and testing of a new method of inducing a standard injury to the calf muscles. Acta Pathol. Microbiol. Scand. A 1975, 83, 259–265. [Google Scholar]
- Knappe, T.; Mittlmeier, T.; Eipel, C.; Amon, M.; Menger, M.D.; Vollmar, B. Effect of systemic hypothermia on local soft tissue trauma-induced microcirculatory and cellular dysfunction in mice. Crit. Care Med. 2005, 33, 1805–1813. [Google Scholar] [CrossRef]
- Crisco, J.J.; Jokl, P.; Heinen, G.T.; Connell, M.D.; Panjabi, M.M. A muscle contusion injury model. Biomechanics, physiology, and histology. Am. J. Sport. Med. 1994, 22, 702–710. [Google Scholar] [CrossRef]
- Schaser, K.-D.; Vollmar, B.; Menger, M.D.; Schewior, L.; Kroppenstedt, S.N.; Raschke, M.; Lübbe, A.S.; Haas, N.P.; Mittlmeier, T. In vivo analysis of microcirculation following closed soft-tissue injury. J. Orthop. Res. 1999, 17, 678–685. [Google Scholar] [CrossRef]
- Griffin, M.J.; Letson, H.L.; Dobson, G.P. Adenosine, lidocaine and Mg2+ (ALM) induces a reversible hypotensive state, reduces lung edema and prevents coagulopathy in the rat model of polymicrobial sepsis. J. Trauma Acute Care Surg. 2014, 77, 471–478. [Google Scholar] [CrossRef]
- Letson, H.L.; Dobson, G.P. Adenosine, lidocaine, and Mg2+ (ALM) resuscitation fluid protects against experimental traumatic brain injury. J. Trauma Acute Care Surg. 2018, 84, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Stratos, I.; Rotter, R.; Eipel, C.; Mittlmeier, T.; Vollmar, B. Granulocyte-colony stimulating factor enhances muscle proliferation and strength following skeletal muscle injury in rats. J. Appl. Physiol. 2007, 103, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; von Roth, P.; Matziolis, G.; Mehta, M.; Perka, C.; Duda, G.N. Dose-response relationship of mesenchymal stem cell transplantation and functional regeneration after severe skeletal muscle injury in rats. Tissue Eng. Part A 2009, 15, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Kee, N.; Sivalingam, S.; Boonstra, R.; Wojtowicz, J.M. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Methods 2002, 115, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.; Pilipowicz, O. Activation of muscle satellite cells in single-fiber cultures. Nitric Oxide 2002, 7, 36–41. [Google Scholar] [CrossRef]
- Ehrhardt, J.; Morgan, J. Regenerative capacity of skeletal muscle. Curr. Opin. Neurol. 2005, 18, 548–553. [Google Scholar] [CrossRef]
- Anderson, J.E. The satellite cell as a companion in skeletal muscle plasticity: Currency, conveyance, clue, connector and colander. J. Exp. Biol. 2006, 209, 2276–2292. [Google Scholar] [CrossRef] [Green Version]
- Dobson, G.P.; Letson, H.L. Far Forward Gaps in Hemorrhagic Shock and Prolonged Field Care: An Update of ALM Fluid Therapy for Field Use. J. Spec. Oper. Med. 2020, 20, 128–134. [Google Scholar] [CrossRef]








| BrdU-Positive Cells [n/mm²] | Ki67-Positive Cells [n/mm²] | TUNEL-Positive Cells [n/mm²] | ||||
|---|---|---|---|---|---|---|
| Day | ALM | Saline | ALM | Saline | ALM | Saline |
| 1 | 11.4 ± 0.01 * | 6.6 ± 0.93 | 5.7 ± 0.95 | 5.3 ± 0.19 | 0.8 ± 0.28 | 0.5 ± 0.09 |
| 4 | 11.5 ± 0.35 * | 7.1 ± 0.63 | 10.6 ± 0.56 | 13.7 ± 1.62 * | 0.3 ± 0.16 | 0.6 ± 0.23 |
| 7 | 8.6 ± 0.45 * | 6.6 ± 0.38 | 11.6 ± 0.26 | 13.0 ± 0.65 | 0.6 ± 0.15 | 0.5 ± 0.14 |
| 14 | 7.2 ± 0.68 * | 5.1 ± 0.39 | 18.5 ± 0.97 * | 14.9 ± 1.41 | 0.4 ± 0.15 | 0.4 ± 0.01 |
| 42 | 3.8 ± 0.84 * | 2.2 ± 0.27 | 17.2 ± 0.25 | 14.2 ± 0.65 | 0.6 ± 0.20 | 0.6 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoeger, N.S.; Mittlmeier, T.; Vollmar, B.; Stratos, I.; Dobson, G.P.; Rotter, R. ALM Therapy Promotes Functional and Histologic Regeneration of Traumatized Peripheral Skeletal Muscle. Biology 2023, 12, 870. https://doi.org/10.3390/biology12060870
Hoeger NS, Mittlmeier T, Vollmar B, Stratos I, Dobson GP, Rotter R. ALM Therapy Promotes Functional and Histologic Regeneration of Traumatized Peripheral Skeletal Muscle. Biology. 2023; 12(6):870. https://doi.org/10.3390/biology12060870
Chicago/Turabian StyleHoeger, Nina Sarah, Thomas Mittlmeier, Brigitte Vollmar, Ioannis Stratos, Geoffrey P. Dobson, and Robert Rotter. 2023. "ALM Therapy Promotes Functional and Histologic Regeneration of Traumatized Peripheral Skeletal Muscle" Biology 12, no. 6: 870. https://doi.org/10.3390/biology12060870