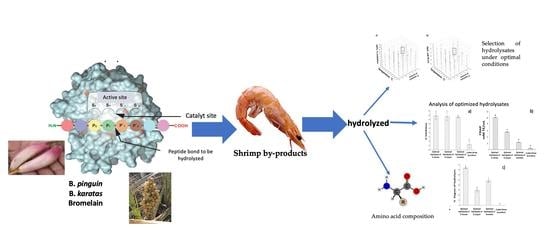
Guamara and Cocuixtle: Source of Proteases for the Transformation of Shrimp By-Products into Hydrolysates with Potential Application
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Optimal Hydrolysis Conditions under a Taguchi L16’ Design
2.2.1. Enzymatic Hydrolysis
2.2.2. Antioxidant Capacity
Inhibition of 2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulphonic Acid Cation Radical (ABTS)
Ferric Reducing Antioxidant Power (FRAP)
2.2.3. Degree of Hydrolysis (DH)
2.3. Aminoacid Profile by GC-MS under Optimal Hydrolysis Conditions
2.4. Statistical Analysis
3. Results
3.1. Antioxidant Capacity
3.1.1. Inhibition of 2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulphonic Acid Cation Radical (ABTS)
3.1.2. Ferric Reducing Antioxidant Power
3.2. Degree of Hydrolysis
- -
- The minimum level set by the STATISTICA program is used if the factor is not significant.
- -
- If only one variable in the factor is significant, this is considered as the optimal condition.
3.3. Analysis of Hydrolysates Obtained under Optimal Hydrolysis Conditions with Proteolytic Extracts of B. pinguin, B. karatas and Bromelain
3.3.1. Selection of Optimal Hydrolysis Conditions with Proteolytic Extract of B. pinguin
3.3.2. Selection of Hydrolyzates under Optimal Conditions with Proteolytic Extract of B. karatas
3.3.3. Selection of Hydrolyzates under Optimal Conditions with Bromelain
3.4. Antioxidant Capacity under Optimal Hydrolysis Conditions
3.4.1. Inhibition of 2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulphonic Acid Cation Radical under Optimal Hydrolysis Conditions
3.4.2. Ferric Reducing Antioxidant Power under Optimal Hydrolysis Conditions
3.5. Degree of Hydrolysis under Optimal Hydrolysis Conditions
3.6. Amino Acid Profile by GC-MS under Optimal Hydrolysis Conditions
4. Discussion
4.1. Antioxidant Capacity
4.1.1. Inhibition of 2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulphonic Acid Cation Radical
4.1.2. Ferric Reducing Antioxidant Power
4.2. Degree of Hydrolysis
4.3. Amino Acid Profile by GC-MS under Optimal Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez, D.E.; Bartoli, C.G.; Grbic, V.; Guiamet, J.J. Vacuolar cysteine proteases of wheat (Triticum aestivum L.) are common to leaf senescence induced by different factors. J. Exp. Bot. 2007, 58, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Hernández, J.M.; Hernández-Mancillas, X.D.; Navarrete, E.L.C.; Mazorra-Manzano, M.; Osuna-Ruiz, I.; Rodríguez-Tirado, V.A.; Salazar-Leyva, J.A. Partial Characterization of the Proteolytic Properties of an Enzymatic Extract From “Aguama” Bromelia pinguin L. Fruit Grown in Mexico. Appl. Biochem. Biotechnol. 2017, 182, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Payrol, J.A.; Mosquera, D.M.G.; Meneses, A.; Cruz, M.D.; Banze, F.; Martínez, M.M.; López, O.R. Determinación de parámetros farmacognósticos y bromatológicos y evaluación de la actividad antiparasitaria de una preparación obtenida del fruto de Bromelia pinguin L. que crece en Cuba. Acta Farm. Bonaer 2005, 24, 377–382. [Google Scholar]
- García-Magaña, M.D.L.; González-Borrayo, J.; Montalvo-González, E.; Rudiño-Piñera, E.; Sáyago-Ayerdi, S.G.; Salazar-Leyva, J.A. Isoelectric focusing, effect of reducing agents and inhibitors: Partial characterization of proteases extracted from Bromelia karatas. Appl. Biol. Chem. 2018, 61, 459–467. [Google Scholar] [CrossRef]
- Rojas, C.M.; Teran, V.; Zuñiga, R.; Caldon, Y. Descripción morfológica de bromelia karatas recurso genético promisorio para Patía, cauca, Colombia. Biotecnol. En El Sect. Agropecu. Y Agroind. BSAA 2014, 12, 62–70. [Google Scholar]
- Efigenia, M.-G.; Miguel, A.-E.L.; Osiris, A.P.M.-O.A.; Alberto, S.-B.J.; de Lourdes, G.-M.M. Physiological and physicochemical behavior of guamara (Bromelia pinguin) and cocuixtle (Bromelia karatas) fruits, as well as the antibacterial effect of their pre-purified proteases. Emir. J. Food Agric. 2021, 33, 277–286. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.C.; Ramón-Sierra, J.; Arias-Argaez, C.; Magaña-Ortiz, D.; Ortiz-Vázquez, E. Antibacterial activity of proteins extracted from the pulp of wild edible fruit of Bromelia pinguin L. Int. J. Food Prop. 2017, 20, 220–230. [Google Scholar] [CrossRef]
- Ragazzo-Sánchez, J.A.; Barrón-Carrillo, D.; Sánchez-Burgos, J.A.; Calderón-Santoyo, M.; Montalvo-González, E.; González-Cruz, E.M.; Barros-Castillo, J.C.; García-Magaña, M.D.L. Utilization of by-products of endemic fruits: Encapsulation of proteolytic extracts of guamara (Bromelia pinguin) and cocuixtle (Bromelia karatas) by electrospraying. LWT 2021, 149, 111670. [Google Scholar] [CrossRef]
- Guimarães-Ferreira, C.A.; Rodrigues, E.G.; Mortara, R.A.; Cabral, H.; Serrano, F.A.; Ribeiro-Dos-Santos, R.; Travassos, L.R. Antitumor Effects In Vitro and In Vivo and Mechanisms of Protection against Melanoma B16F10-Nex2 Cells By Fastuosain, a Cysteine Proteinase from Bromelia fastuosa. Neoplasia 2007, 9, 723–733. [Google Scholar] [CrossRef]
- Aguilera-Aguirre, S.; Meza-Espinoza, L.; Hernández-Mendoza, A.; Vallejo-Córdoba, B.; González-Córdova, A.F.; Montalvo-González, E. Evaluación de la capacidad de inhibición de hemólisis oxidativa y actividad antimicrobiana de fracciones peptídicas obtenidas de la hidrólisis de proteínas de huevo, leche y soya usando proteasas extraídas de Bromelia pinguin y Bromelia karatas. TIP Rev. Espec. En Cienc. Químico-Biológicas 2018, 21, 13–21. [Google Scholar] [CrossRef]
- Meza-Espinoza, L.; Vivar-Vera, M.D.L.; García-Magaña, M.D.L.; Sáyago-Ayerdi, S.G.; Chacón-López, A.; Becerrea-Verdín, E.M.; Montalvo-González, E. Enzyme activity and partial characterization of proteases obtained from Bromelia karatas fruit and compared with Bromelia pinguin proteases. Food Sci. Biotechnol. 2018, 27, 509–517. [Google Scholar] [CrossRef]
- Romero-Garay, M.G.; Martínez-Montaño, E.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Montalvo-González, E.; García-Magaña, M.D.L. Bromelia karatas and Bromelia pinguin: Sources of plant proteases used for obtaining antioxidant hydrolysates from chicken and fish by-products. Appl. Biol. Chem. 2020, 63, 1–11. [Google Scholar] [CrossRef]
- Prospitti, A.; Cancelarich, L.N.; Perrando, J.; Natalucci, C.L.; Pardo, M.F. Balansain R, a new proteolytic preparation for the production of antioxidant peptides from bovine whey. Lat. Am. J. Pharm. 2015, 34, 1387–1395. [Google Scholar]
- Bruno, M.A.; Lazza, C.M.; Errasti, M.E.; López, L.M.; Caffini, N.O.; Pardo, M.F. Milk clotting and proteolytic activity of an enzyme preparation from Bromelia hieronymi fruits. LWT-Food Sci. Technol. 2010, 43, 695–701. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S. Trends in shrimp processing waste utilization: An industrial prospective. Trends Food Sci. Technol. 2020, 103, 20–35. [Google Scholar] [CrossRef]
- Senphan, T.; Benjakul, S.; Kishimura, H. Characteristics and antioxidative activity of carotenoprotein from shells of Pacific white shrimp extracted using hepatopancreas proteases. Food Biosci. 2014, 5, 54–63. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Romero-Garay, M.G.; Montalvo-González, E.; Hernández-González, C.; Soto-Domínguez, A.; Becerra-Verdín, E.M.; García-Magaña, M.D.L. Bioactivity of peptides obtained from poultry by-products: A review. Food Chem. X 2022, 13, 100181. [Google Scholar] [CrossRef]
- Bechaux, J.; Gatellier, P.; Le Page, J.-F.; Drillet, Y.; Sante-Lhoutellier, V. A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food Funct. 2019, 10, 6244–6266. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 2021, 147, 110468. [Google Scholar] [CrossRef]
- Dullius, A.; Fassina, P.; Giroldi, M.; Goettert, M.I.; de Souza, C.F.V. A biotechnological approach for the production of branched chain amino acid containing bioactive peptides to improve human health: A review. Food Res. Int. 2020, 131, 109002. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Carvajal, C.; Márquez, M.; Báez, R.; Morris, H.; Santos, R.; de los Ángeles Chávez, M. Obtención de Preparados Enzimáticos a Partir de Tallos de Piña (Ananas Comosus) Potencialidades de uso en la Biotecnología y la Medicina. Rev. CENIC Cienc. Biológicas 2005, 36, 1–12. [Google Scholar]
- Kim, S.K. Marine Proteins and Peptides: Biological Activities and Applications; John Wiley & Sons, Ltd.: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Brion-Espinoza, I.A.; Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Calderón-Chiu, C.; Calderón-Santoyo, M. Edible pectin film added with peptides from jackfruit leaves obtained by high-hydrostatic pressure and pepsin hydrolysis. Food Chem. X 2021, 12, 100170. [Google Scholar] [CrossRef]
- Roslan, J.; Yunos, K.F.M.; Abdullah, N.; Kamal, S.M.M. Characterization of Fish Protein Hydrolysate from Tilapia (Oreochromis Niloticus) by-Product. Agric. Agric. Sci. Procedia 2014, 2, 312–319. [Google Scholar] [CrossRef]
- Stauffer, C.E. Principles of enzymology for the food sciences (2nd edn): By John R. Whitaker, Marcel Dekker, 1993. 185 00 (xv + 625 pages) ISBN 0 8247 9148 7. Trends Food Sci. Technol. 1994, 5, 304–305. [Google Scholar] [CrossRef]
- Baez-Suarez, A.J.; Ospina-De-Barreneche, N.; Zapata-Montoya, J.E. Efecto de Temperatura, pH, Concentración de Sustrato y Tipo de Enzima en la Hidrólisis Enzimática de Vísceras de Tilapia Roja (Oreochromis spp.). Inf. Tecnol. 2016, 27, 63–76. [Google Scholar] [CrossRef]
- Wan, M.-Y.; Dong, G.; Yang, B.-Q.; Feng, H. Identification and characterization of a novel antioxidant peptide from feather keratin hydrolysate. Biotechnol. Lett. 2016, 38, 643–649. [Google Scholar] [CrossRef]
- Djellouli, M.; López-Caballero, M.E.; Arancibia, M.Y.; Karam, N.; Martínez-Alvarez, O. Antioxidant and Antimicrobial Enhancement by Reaction of Protein Hydrolysates Derived from Shrimp By-Products with Glucosamine. Waste Biomass Valorization 2020, 11, 2491–2505. [Google Scholar] [CrossRef]
- Yang, Q.; Cai, X.; Yan, A.; Tian, Y.; Du, M.; Wang, S. A specific antioxidant peptide: Its properties in controlling oxidation and possible action mechanism. Food Chem. 2020, 327, 126984. [Google Scholar] [CrossRef]
- Fillería, S.G.; Nardo, A.E.; Paulino, M.; Tironi, V. Peptides derived from the gastrointestinal digestion of amaranth 11S globulin: Structure and antioxidant functionality. Food Chem. Mol. Sci. 2021, 3, 100053. [Google Scholar] [CrossRef]
- Jain, S.; Anal, A.K. Production and characterization of functional properties of protein hydrolysates from egg shell membranes by lactic acid bacteria fermentation. J. Food Sci. Technol. 2017, 54, 1062–1072. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Li, J.; Sun, H.; Liu, Y. Physicochemical and antioxidative characteristics of black bean protein hydrolysates obtained from different enzymes. Food Hydrocoll. 2019, 97, 105222. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Zhang, X.; Duan, M.; Jia, L.; Xu, H.; Liang, M.; Liu, J. Fish protein hydrolysate supplementation in plant protein based diets for tiger puffer (Takifugu rubripes) is an effective strategy of fish meal sparing. Aquac. Rep. 2021, 20, 100720. [Google Scholar] [CrossRef]
- Nikoo, M.; Xu, X.; Regenstein, J.M.; Noori, F. Autolysis of Pacific white shrimp (Litopenaeus vannamei) processing by-products: Enzymatic activities, lipid and protein oxidation, and antioxidant activity of hydrolysates. Food Biosci. 2021, 39, 100844. [Google Scholar] [CrossRef]
- Karimi, N.; Nikoo, M.; Gavlighi, H.A.; Gheshlaghi, S.P.; Regenstein, J.M.; Xu, X. Effect of pacific white shrimp (Litopenaeus vannamei) protein hydrolysates (SPH) and (−)-epigallocatechin gallate (EGCG) on sourdough and bread quality. LWT-Food Sci. Technol. 2020, 131, 109800. [Google Scholar] [CrossRef]
- Lima-Cabello, E.; Morales-Santana, S.; Foley, R.C.; Melser, S.; Alché, V.; Siddique, K.H.; Singh, K.B.; Alché, J.D.; Jimenez-Lopez, J.C. Ex vivo and in vitro assessment of anti-inflammatory activity of seed β-conglutin proteins from Lupinus angustifolius. J. Funct. Foods 2018, 40, 510–519. [Google Scholar] [CrossRef]
- Gomez, L.; Figueroa, O.A.; Zapata, J.E. Actividad Antioxidante de Hidrolizados Enzimáticos de Plasma Bovino Obtenidos por Efecto de Alcalasa® 2.4 L. Inf. Tecnol. 2013, 24, 33–42. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; O Abioye, R.; Okagu, I.U.; I Obeme-Nmom, J. Bioaccessibility of bioactive peptides: Recent advances and perspectives. Curr. Opin. Food Sci. 2021, 39, 182–189. [Google Scholar] [CrossRef]
- Mai, K.; Xue, M.; He, G.; Xie, S.; Kaushik, S.J. Chapter 4-Protein and amino acids. In Fish Nutrition, 4th ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 181–302. [Google Scholar] [CrossRef]
- Wu, D.; Li, M.; Ding, J.; Zheng, J.; Zhu, B.; Lin, S. Structure-activity relationship and pathway of antioxidant shrimp peptides in a PC12 cell model. J. Funct. Foods 2020, 70, 103978. [Google Scholar] [CrossRef]
- Dayakar, B.; Xavier, K.M.; Ngasotter, S.; Layana, P.; Balange, A.K.; Priyadarshini, B.; Nayak, B.B. Characterization of spray-dried carotenoprotein powder from Pacific white shrimp (Litopenaeus vannamei) shells and head waste extracted using papain: Antioxidant, spectroscopic, and microstructural properties. LWT 2022, 159, 113188. [Google Scholar] [CrossRef]
- Tonon, R.V.; dos Santos, B.A.; Couto, C.C.; Mellinger-Silva, C.; Brígida, A.I.S.; Cabral, L.M. Coupling of ultrafiltration and enzymatic hydrolysis aiming at valorizing shrimp wastewater. Food Chem. 2016, 198, 20–27. [Google Scholar] [CrossRef]
- Johnson, K.A.; Goody, R.S. The Original Michaelis Constant: Translation of the 1913 Michaelis–Menten Paper. Biochemistry 2011, 50, 8264–8269. [Google Scholar] [CrossRef]
- Kurozawa, L.; Park, K.J.; Hubinger, M. Optimization of the Enzymatic Hydrolysis of Chicken Meat Using Response Surface Methodology. J. Food Sci. 2008, 73, C405–C412. [Google Scholar] [CrossRef]
- Haard, N.F. A Review of Proteotlytic Enzymes from Marine Organisms and Their Application in the Food Industry. J. Aquat. Food Prod. Technol. 1992, 1, 17–35. [Google Scholar] [CrossRef]
- Ketnawa, S.; Martínez-Alvarez, O.; Gómez-Estaca, J.; Gómez-Guillén, M.D.C.; Benjakul, S.; Rawdkuen, S. Obtaining of functional components from cooked shrimp (Penaeus vannamei) by enzymatic hydrolysis. Food Biosci. 2016, 15, 55–63. [Google Scholar] [CrossRef]
- Islam, S.; Wang, H.; Admassu, H.; Sulieman, A.A.; Wei, F.A. Health benefits of bioactive peptides produced from muscle proteins: Antioxidant, anti-cancer, and anti-diabetic activities. Process. Biochem. 2022, 116, 116–125. [Google Scholar] [CrossRef]
- Meza-Espinoza, L.; García-Magaña, M.D.L.; Vivar-Vera, M.D.L.; Sáyago-Ayerdi, S.G.; Chacón-López, A.; Becerra-Verdín, E.M.; Muy-Rangel, M.D.; Montalvo-González, E. Aspectos etnobotánicos, nutricionales y actividad biológica de extractos de frutos del género bromelia. Rev. Fitotec. Mex. 2017, 40, 425–437. [Google Scholar] [CrossRef]
- Tea, I.; Tcherkez, G. Natural Isotope Abundance in Metabolites: Techniques and Kinetic Isotope Effect Measurement in Plant, Animal, and Human Tissues, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 596. [Google Scholar] [CrossRef]
- Hunt, S. Degradation of Amino Acids Accompanying in vitro Protein Hydrolysis. In Chemistry and Biochemistry of the Amino Acids; Barrett, G.C., Ed.; Springer: Dordrecht, The Netherlands, 1985; pp. 376–398. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Shahidi, F. Bioactive peptides from shrimp shell processing discards: Antioxidant and biological activities. J. Funct. Foods 2017, 34, 7–17. [Google Scholar] [CrossRef]
- Venuste, M.; Zhang, X.; Shoemaker, C.F.; Karangwa, E.; Abbas, S.; Kamdem, P.E. Influence of enzymatic hydrolysis and enzyme type on the nutritional and antioxidant properties of pumpkin meal hydrolysates. Food Funct. 2013, 4, 811–820. [Google Scholar] [CrossRef]
- Silverman, S.N.; Phillips, A.A.; Weiss, G.M.; Wilkes, E.B.; Eiler, J.M.; Sessions, A.L. Practical considerations for amino acid isotope analysis. Org. Geochem. 2022, 164, 104345. [Google Scholar] [CrossRef]



| Treatment | Factors | ||||
|---|---|---|---|---|---|
| pH | Temperature, °C | Time, h | Substrate, g | Enzyme, µg/mL | |
| 1 | 6.5 | 25 | 0.5 | 0.5 | 50 |
| 2 | 6.5 | 30 | 1 | 0.75 | 100 |
| 3 | 6.5 | 35 | 2 | 1 | 150 |
| 4 | 6.5 | 40 | 4 | 1.5 | 250 |
| 5 | 7.0 | 25 | 1 | 1.5 | 150 |
| 6 | 7.0 | 30 | 0.5 | 1 | 250 |
| 7 | 7.0 | 35 | 4 | 0.75 | 50 |
| 8 | 7.0 | 40 | 2 | 0.5 | 100 |
| 9 | 7.5 | 25 | 2 | 0.75 | 250 |
| 10 | 7.5 | 30 | 4 | 0.5 | 150 |
| 11 | 7.5 | 35 | 0.5 | 1.5 | 100 |
| 12 | 7.5 | 40 | 1 | 1 | 50 |
| 13 | 8.0 | 25 | 4 | 1 | 100 |
| 14 | 8.0 | 30 | 2 | 1.5 | 50 |
| 15 | 8.0 | 35 | 1 | 0.5 | 250 |
| 16 | 8.0 | 40 | 0.5 | 0.75 | 150 |
| Treatment | ABTS, % Inhibition | ||
|---|---|---|---|
| B. karatas | B. pinguin | Bromelain | |
| 1 | 71.0 ± 1.1 ab | 77.7 ± 1.9 ab | 66.27 ± 2.4 a |
| 2 | 75.6 ± 0.9 cde | 78.6 ± 2.6 bcd | 76.9 ± 0.9 a |
| 3 | 76.5 ± 0.2 cde | 81.9 ± 2.0 cde | 77.0 ± 0.7 c |
| 4 | 82.5 ± 0.2 f | 82.5 ± 1.6 de | 77.0 ± 1.3 c |
| 5 | 78.3 ± 1.4 de | 80.6 ± 1.2 bcde | 66.4 ± 4.7 c |
| 6 | 77.5 ± 0.3 de | 82.7 ± 0.5 e | 76.4 ± 1.6 bc |
| 7 | 72.6 ± 0.1 ab | 82.2 ± 1.4 cde | 65.8 ± 3.6 bc |
| 8 | 73.5 ± 0.7 cd | 80.2 ± 1.0 bcde | 79.4 ± 0.3 c |
| 9 | 73.3 ± 0.6 bc | 78.3 ± 1.5 bc | 75.8 ± 3.8 c |
| 10 | 69.9 ± 0.3 a | 79.9 ± 0.8 ab | 77.0 ± 2.1 a |
| 11 | 79.5 ± 2.3 ef | 81.2 ± 1.5 bcde | 76.5 ± 3.5 c |
| 12 | 78.1 ± 1.0 de | 78.2 ± 1.1 bc | 65.8 ± 0.5 c |
| 13 | 76.1 ± 0.0 cd | 78.5 ± 1.0 bcd | 79.2 ± 1.5 c |
| 14 | 75.3 ± 0.5 cd | 76.9 ± 1.6 a | 76.4 ± 2.4 c |
| 15 | 77.0 ± 2.4 cde | 77.2 ± 4.3 ab | 78.5 ± 0.1 a |
| 16 | 79.2 ± 1.2 cde | 80.2 ± 0.7 bcde | 67.1 ± 0.8 ab |
| Treatment | Ferric Reducing Antioxidant Power, mM TE/mL | ||
|---|---|---|---|
| B. karatas | B. pinguin | Bromelain | |
| 1 | 1.31 ± 0.04 ab | 4.06 ± 1.07 a | 0.23 ± 0.01 a |
| 2 | 1.46 ± 0.05 ab | 4.17 ± 0.44 ab | 1.48 ± 0.003 ab |
| 3 | 2.08 ± 0.05 bcde | 4.14 ± 1.23 abcd | 0.48 ± 0.07 cd |
| 4 | 2.77 ± 0.03 f | 5.31 ± 0.14 cde | 1.15 ± 0.04 f |
| 5 | 2.61 ± 0.17 ef | 5.00 ± 0.39 abcde | 0.65 ± 0.01 def |
| 6 | 2.76 ± 0.37 cde | 5.70 ± 0.51 de | 1.26 ± 0.003 cd |
| 7 | 1.43 ± 0.02 a | 4.59 ± 015 abcde | 0.45 ± 0.02 ab |
| 8 | 2.12 ± 0.15 bcde | 4.31 ± 0.08 abcd | 1.26 ± 0.01 a |
| 9 | 1.97 ± 0.04 abcd | 4.85 ± 0.11 bcde | 0.87 ± 0.02 a |
| 10 | 1.66 ± 0.14 abc | 5.01 ± 0.21 abcde | 0.74 ± 0.01 a |
| 11 | 2.58 ± 0.28 de | 5.99 ± 0.52 e | 1.35 ± 0.06 ef |
| 12 | 1.58 ± 0.05 abc | 5.44 ± 0.34 cde | 0.46 ± 0.11 cde |
| 13 | 1.82 ± 0.04 bd | 4.72 ± 0.53 abc | 1.82 ± 0.19 bc |
| 14 | 2.54 ± 0.21 e | 5.32 ± 0.89 abcde | 0.29 ± 0.06 cde |
| 15 | 1.68 ± 0.13 ab | 4.39 ± 0.28 abcd | 1.13 ± 0.007 a |
| 16 | 1.47 ± 0.22 abc | 5.34 ± 0.73 cde | 0.48 ± 105.0 ab |
| Treatment | Degree of Hydrolysis | ||
|---|---|---|---|
| B. karatas | B. pinguin | Bromelain | |
| 1 | 25.4 ± 1.0 h | 15.8 ± 0.8 e | 20.9 ± 0.5 fg |
| 2 | 15.2 ± 1.0 e | 18.7 ± 0.7 f | 22.1 ± 1.2 g |
| 3 | 26.0 ± 0.2 h | 16.4 ± 0.6 ef | 3.0 ± 0.5 abcd |
| 4 | 12.6 ± 1.0 d | 6.2 ± 0.8 c | 3.2 ± 0.5 abc |
| 5 | 9.9 ± 1.1 c | 3.8 ± 0.8 b | 22.2 ± 1.4 ab |
| 6 | 16.7 ± 0.8 f | 25 ± 0.6 b | 2.2 ± 0.3 g |
| 7 | 6.4 ± 0.7 b | 10.2 ± 0.3 d | 91.9 ± 1.1 cd |
| 8 | 88.1 ± 0.5 i | 48.9 ± 1.4 h | 5.9 ± 0.7 k |
| 9 | 14.2 ± 0.8 e | 71.3 ± 1.3 j | 0.6 ± 0.1 f |
| 10 | 10.0 ± 0.4 c | 59.3 ± 3.0 i | 68.4 ± 1.7 i |
| 11 | 1.3 ± 0.1 a | 3.5 ± 0.6 ab | 6.1 ± 0.7 a |
| 12 | 19.1 ± 0.8 g | 16.0 ± 0.2 e | 64.3 ± 0.6 j |
| 13 | 1.1 ± 0.2 a | 0.7 ± 02 a | 14.7 ± 1.0 d |
| 14 | 14.4 ± 1.2 e | 3.6 ± 0.6 b | 21.7 ± 1.7 bcd |
| 15 | 26.3 ± 1.3 g | 3.1 ± 0.5 b | 4.9 ± 0.4 h |
| 16 | 12.1 ± 0.2 d | 41.8 ± 0.3 g | 18.4 ± 1.6 e |
| Factor | Degree of Hydrolysis | ABTS | FRAP | Optimum |
|---|---|---|---|---|
| pH | 8 | 8 | 8 | 8 |
| Temperature, °C | 35 | 30 | 35 | 30 |
| Hydrolysis time, h | 0.5 | 0.5 | 0.5 | 0.5 |
| Substrate amount, g | 1 | 1 | 1 | 1 |
| Enzyme amount, µg/mL | 100 | 250 | 100 | 100 |
| Factor | Degree of Hydrolysis | ABTS | FRAP | Optimum |
|---|---|---|---|---|
| pH | 8 | 7.5 | 7.5 | 7.5 |
| Temperature, °C | 40 | 40 | 35 | 40 |
| Hydrolysis time, h | 4 | 1 | 0.5 | 0.5 |
| Substrate amount, g | 1 | 0.5 | 0.5 | 0.5 |
| Enzyme amount, µg/mL | 50 | 100 | 100 | 100 |
| Factor | Degree of Hydrolysis | ABTS | FRAP | Optimum |
|---|---|---|---|---|
| Hydrolysis time, h | 1 | 1 | 4 | 1 |
| Substrate amount, g | 0.5 | 1.5 | 0.75 | 1.5 |
| Enzyme amount, µg/mL | 50 | 50 | 100 | 100 |
| Amino Acids (g of Amino Acid/100 g of Protein) | GC-MS | Requirements According to the FAO (1991) (g of Amino Acid/100 g of Protein) | |||||
|---|---|---|---|---|---|---|---|
| Shrimp By-Products | B. karatas | B. pinguin | Bromelain | Children | Adults | ||
| Essential | Isoleucine | 8.8 | 9.9 | 7.9 | 7.3 | 2.8 | 1.3 |
| Leucine | 13.9 | 20.5 | 13.8 | 12.4 | 6.6 | 1.9 | |
| Lysine | ND | ND | ND | ND | 5.8 | 1.6 | |
| Tryptophan | ND | ND | ND | ND | 1.1 | 0.5 | |
| Histidine | ND | ND | ND | ND | 1.9 | 1.6 | |
| Threonine | 4.3 | 5.4 | 7.1 | 6.4 | 1.4 | 0.9 | |
| Valine | 12.0 | 14.6 | 11.4 | 11.2 | 3.5 | 1.3 | |
| Methionine 1 | 2.4 | 2.0 | 1.8 | 2.2 | 2.5 | 1.7 | |
| Phenylalanine 2 | 7.9 | 8.9 | 9.5 | 11.3 | 6.3 | 1.9 | |
| Non-essential | Aspartic Acid | 5.7 | 8.9 | 9.5 | 11.3 | ||
| Glutamic acid | 8.0 | 9.8 | 13.1 | 17.6 | |||
| Serine | 3.5 | 2.9 | 5.2 | 5.7 | |||
| Glycin | 10.5 | 2.5 | 2.2 | 2.0 | |||
| Arginine | ND | ND | ND | ND | |||
| Alanin | 14.0 | 11.2 | 14.8 | 11.5 | |||
| Proline | 8.7 | 4.7 | 8.0 | 4.6 | |||
| % Amino acid distribution | HAA | 67.8 | 70.4 | 62.8 | 57.1 | ||
| AAA | 7.9 | 7.5 | 5.2 | 7.8 | |||
| EAA | 49.5 | 59.9 | 47.2 | 47.3 | |||
| NCAA | 21.7 | 27.1 | 34.9 | 41.0 | |||
| PCAA | ND | ND | ND | ND | |||
| BCAAs | 34.8 | 44.9 | 33.1 | 30.9 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Jiménez, J.M.d.J.; Montalvo-González, E.; López-García, U.M.; Barros-Castillo, J.C.; Ragazzo-Sánchez, J.A.; García-Magaña, M.d.L. Guamara and Cocuixtle: Source of Proteases for the Transformation of Shrimp By-Products into Hydrolysates with Potential Application. Biology 2023, 12, 753. https://doi.org/10.3390/biology12050753
Rodríguez-Jiménez JMdJ, Montalvo-González E, López-García UM, Barros-Castillo JC, Ragazzo-Sánchez JA, García-Magaña MdL. Guamara and Cocuixtle: Source of Proteases for the Transformation of Shrimp By-Products into Hydrolysates with Potential Application. Biology. 2023; 12(5):753. https://doi.org/10.3390/biology12050753
Chicago/Turabian StyleRodríguez-Jiménez, Juan Miguel de Jesús, Efigenia Montalvo-González, Ulises Miguel López-García, Julio César Barros-Castillo, Juan Arturo Ragazzo-Sánchez, and María de Lourdes García-Magaña. 2023. "Guamara and Cocuixtle: Source of Proteases for the Transformation of Shrimp By-Products into Hydrolysates with Potential Application" Biology 12, no. 5: 753. https://doi.org/10.3390/biology12050753