Antioxidant, Anti-Inflammatory and Anti-Obesity Potential of Extracts Containing Phenols, Chlorophyll and Carotenoids from Mexican Wild Populations of Bacopa monnieri (L.) Wettst
Abstract
:Simple Summary
Abstract
1. Introduction
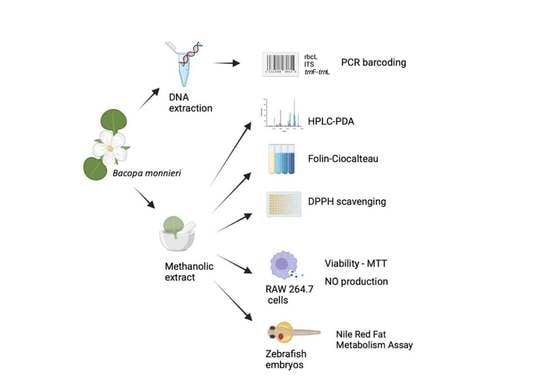
2. Materials and Methods
2.1. Materials
2.2. Molecular Identification
2.3. Preparation of Extracts
2.4. Determination of Carotenoids and Chlorophylls Profile using HPLC-PDA
2.5. Total Phenolic Content
2.6. Determination of DPPH• Scavenging
2.7. Cell Assays
2.8. Toxicity
2.9. Anti-Inflammatory Potential
2.10. Zebrafish Nile Red Fat Metabolism Assay
2.11. Statistical Analysis
3. Results
3.1. Identification of Bacopa monnieri using Molecular Data
3.2. Carotenoids and Chlorophylls Profiling
3.3. Total Phenolic Content
3.4. Antioxidant Potential
3.5. Anti-Inflammatory Potential
3.6. Lipid-Reducing Activity in Different Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tropicos.org. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 30 January 2023).
- Kean, J.D.; Downey, L.A.; Stough, C. Systematic Overview of Bacopa monnieri (L.) Westtst. Dominant Poly-Herbal Formulas in Children and Adolescents. Medicines 2017, 4, 86. [Google Scholar] [CrossRef]
- Ritter, S.; Urmann, C.; Herzog, R.; Glaser, J.; Bieringer, S.; Geisberger, T.; Eisenreich, W.; Riepl, H. Where is Bacosine in Commercially Available Bacopa monnieri? Planta Med. 2020, 86, 565–570. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, F.; Lee, S.; Kang, S.S.; Shin, H.S. Selected commercial plants: A review of extraction and isolation of bioactive compounds and their pharmacological market value. Trends Food Sci. Technol. 2018, 82, 89–109. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Farooqui, T.; Madan, A.; Ong, J.H.-J.; Ong, W.-Y. Ayurvedic Medicine for the treatment of Dementia: Mechanistic Aspect. Evid. Based Complement. Alternat. Med. 2018, 2018, 2481076. [Google Scholar] [CrossRef] [PubMed]
- Deepak, M.; Sangli, G.K.; Arun, P.C.; Amit, A. Quantitative determination of major saponin mixture bacoside A in Bacopa monnieri by HPLC. Phytochem. Anal. 2005, 16, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.; Channa, S. Relaxant effect of ethanol extract of Bacopa monnieri on trachea, pulmonary artery and aorta from rabbit and guinea pig. Phytother. Res. 1997, 11, 323–325. [Google Scholar] [CrossRef]
- Allan, J.J.; Damodaran, A.; Deshmukh, N.S.; Goudar, K.S.; Amit, A. Safety evaluation of a standardized phytochemical composition extracted from Bacopa monnieri in Sprague-Dawley rats. Food Chem. Toxicol. 2007, 45, 1928–1937. [Google Scholar] [CrossRef]
- KeenMind® and CDRI08®, Soho FLORDISTM International. Available online: https://www.flordis.com.au/products/keenmind/ (accessed on 30 January 2023).
- CBOL Plant Working Group. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef] [PubMed]
- Taberlet, P.; Coissac, E.; Pompanon, F.; Gielly, L.; Miquel, C.; Valentini, A.; Vermat, T.; Corthier, G.; Brochmann, C.; Willerslev, E. Power and limitations of the chloroplast trnL (UAA) intron for plant DNA barcoding. Nucleic Acids Res. 2007, 35, e14. [Google Scholar] [CrossRef]
- Kress, W.J.; Wurdack, K.J.; Zimmer, E.A.; Weigt, L.A.; Janzen, D.H. Use of DNA barcodes to identify flowering plants. Proc. Natl. Acad. Sci. USA 2005, 102, 8369–8374. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor, J.L. Catálogo de las plantas vasculares nativas de México. Rev. Mex. Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- Christopher, C.; Johnson, A.J.; Mathew, P.J.; Baby, S. Elite genotypes of Bacopa monnieri, with high contents of Bacoside A and Bacopaside I, from southern Western Ghats in India. Ind. Crop. Prod. 2017, 98, 76–81. [Google Scholar] [CrossRef]
- Mathur, S.; Sharma, S.; Gupta, M.M.; Kumar, S. Evaluation of the Indian germplasm collection of the medicinal plant Bacopa monnieri (L) Pennell by use of multivariate approaches. Euphytica 2003, 133, 255–265. [Google Scholar] [CrossRef]
- Giuntella, O.; Rieger, M.; Rotunno, L. Weight gains from trade in foods: Evidence from Mexico. J. Int. Econ. 2020, 122, 103277. [Google Scholar] [CrossRef]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff; New York Botanical Garden’s Virtual Herbarium: New York, NY, USA, 2020; Available online: http://sweetgum.nybg.org/science/ih (accessed on 30 January 2023).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, N., Gelfand, D., Sninsky, J., White, T., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primer for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef]
- Kenneth, G.K.; McCourt, R.M.; Cimino, M.T.; Delwiche, C.F. The closest living relatives of land plants. Science 2001, 294, 2351–2353. [Google Scholar]
- Lopes, G.; Clarinha, D.; Vasconcelos, V. Carotenoids from cyanobacteria: A biotechnological approach for the topical treatment of psoriasis. Microorganisms 2020, 8, 302. [Google Scholar] [CrossRef]
- Morone, J.; Lopes, G.; Preto, M.; Vasconcelos, V.; Martins, R. Exploitation of Filamentous and Picoplanktonic Cyanobacteria for Cosmetic Applications: Potential to Improve Skin Structure and Preserve Dermal Matrix Components. Mar. Drugs 2020, 18, 486. [Google Scholar] [CrossRef]
- Matthaus, B. Antioxidant activity of extracts obtained from residues of different oilseeds. J. Agric. Food Chem. 2002, 50, 3444–3452. [Google Scholar] [CrossRef]
- Barbosa, M.; Lopes, G.; Ferreres, F.; Andrade, P.B.; Pereira, D.M.; Gil-Izquierdo, Á.; Valentao, P. Phlorotannin extracts from Fucales: Marine polyphenols as bioregulators engaged in inflammation-related mediators and enzymes. Algal Res. 2017, 28, 1–8. [Google Scholar] [CrossRef]
- Urbatzka, R.; Freitas, S.; Palmeira, A.; Almeida, T.; Moreira, J.; Azevedo, C.; Afonso, C.; Correia-da-Silva, M.; Sousa, E.; Pinto, M.; et al. Lipid reducing activity and toxicity profiles of a library of polyphenol derivatives. Eur. J. Med. Chem. 2018, 151, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Tungphatthong, C.; Somnuek, J.; Phadungcharoen, T.; Ingkaninan, K.; Denduang, J.; Sukrong, S. DNA barcoding of species of Bacopa coupled with high—Resolution melting analysis. Genome 2018, 61, E867–E877. [Google Scholar] [CrossRef] [PubMed]
- BOLDSYSTEMS. Available online: https://www.boldsystems.org/index.php/Public_RecordView?processid=GBVX6397-15 (accessed on 16 April 2023).
- Britton, G.; Khachik, F. Carotenoids in Food. In Carotenoids 5; Britton, G., Pfander, H., Liaaen-Jensen, S., Eds.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 2009; pp. 45–66. [Google Scholar]
- Dhami, N.; Cazzonelli, C.I. Environmental impacts on carotenoid metabolism in leaves. Plant Growth Regul. 2020, 92, 455–477. [Google Scholar] [CrossRef]
- Dhami, N.; Tissue, D.T.; Cazzonelli, C.I. Leaf-age dependent response of carotenoid accumulation to elevated CO2 in Arabidopsis. Arch. Biochem. Biophys. 2018, 647, 67–75. [Google Scholar] [CrossRef]
- Mihaylova, D.; Vrancheva, R.; Petkova, N.; Ognyanov, M.; Desseva, I.; Ivanov, I.; Popova, M.; Popova, A. Carotenoids, tocopherols, organic acids, carbohydrate, and mineral content in different medicinal plant extracts. Z. Naturforsch. C 2018, 73, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, F.; Paredes-López, O. Natural Colorants for Food and Nutraceutical Uses; CRC Press: Boca Raton, FL, USA, 2003; pp. 133–166. [Google Scholar]
- Moreno-Escobar, J.A.; Bazaldúa, S.; Villarreal, M.L.; Bonilla-Barbosa, J.R.; Mendoza, S.; Rodríguez-López, V. Cytotoxic and antioxidant activities of selected Lamiales species from Mexico. Pharm. Biol. 2011, 49, 1243–1248. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Jain, C.K.; Mathur, A. Comparative analysis of saponins, flavonoids, phenolics and antioxidant activities of field acclimatized and in vitro propagated Bacopa monnieri (L.) Pennell from different location in India. Indian J. Exp. Biol. 2019, 57, 259–268. [Google Scholar]
- Radha, P.; Padma, P.R. Antioxidant status and radical scavenging effects of Bacopa monnieri (L.) Pennell. Ann. Phytomed. 2016, 5, 104–109. [Google Scholar]
- Rafi, M.M.; Shafaie, Y. Dietary lutein modulates inducible nitric oxide synthase (iNOS) gene and protein expression in mouse macrophage cells (RAW 264.7). Mol. Nutr. Food Res. 2007, 51, 333–340. [Google Scholar] [CrossRef]
- Saini, N.; Singh, D.; Sandhir, R. Bacopa monnieri prevents colchicine-induced dementia by anti-inflammatory action. Metab. Brain Dis. 2019, 34, 505–518. [Google Scholar] [CrossRef]
- Sireeratawong, S.; Jaijoy, K.; Khonsung, P.P.; Lertprasertsuk, N.; Ingkaninan, K. Acute and chronic toxicities of Bacopa monnieri extract in Sprague-Dawley rats. BMC Complement. Altern. Med. 2016, 16, 249. [Google Scholar] [CrossRef] [PubMed]
- Dooley, K.; Zon, L.I. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, K.-J.; Kim, B.-H.; Koh, E.-J.; Seo, M.-J.; Lee, B.-Y. 6-Gingerol Suppresses Adipocyte-Derived mediators of inflammation in vitro and in High-Fat Diet-Induced Obese Zebra fish. Planta Med. 2017, 83, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.; Gonçalves, S.N.; Sousa, M.L.; Ribeiro, T.; Rosa, F.; Leão, P.N.; Vasconcelos, V.; Alves, R.M.; Urbatzka, R. Chlorophyll derivatives from marine cyanobacteria with lipid-reducing activities. Mar. Drugs. 2019, 17, 229. [Google Scholar] [CrossRef] [PubMed]
- Mounien, L.; Tourniaire, F.; Landrier, J.-F. Anti-Obesity Effect of Carotenoids: Direct Impact on Adipose Tissue and Adipose Tissue-Driven Indirect Effects. Nutrients 2019, 11, 1562. [Google Scholar] [CrossRef] [PubMed]
- Villa-Rivera, M.G.; Ochoa-Alejo, N. Chili pepper Carotenoids: Nutraceutical properties and mechanisms of action. Molecules 2020, 25, 5573. [Google Scholar] [CrossRef]
- Costa, M.; Rosa, F.; Ribeiro, T.; Hernández-Bautista, R.; Bonaldo, M.; Gonçalves Silva, N.; Eiríksson, F.; Thorsteinsdóttir, M.; Ussar, S.; Urbatzka, R. Identification of cyanobacterial strains with potential for the treatment of obesity-related co-morbidities by bioactivity, toxicity evaluation and metabolite profiling. Mar. Drugs 2019, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Bellver, M.; Costa, S.L.D.; Sánchez, B.A.; Vasconcelos, V.; Urbatzka, R. Inhibition of intestinal lipid absorption by cyanobacterial strains in zebrafish larvae. Mar. Drugs 2021, 19, 161. [Google Scholar] [CrossRef] [PubMed]
- Bel Mabrouk, S.; Reis, M.; Sousa, M.L.; Ribeiro, T.; Almeida, J.R.; Pereira, S.; Antunes, J.; Rosa, F.; Vasconcelos, V.; Achour, L.; et al. The marine seagrass Halophila stipulacea as a source of bioactive metabolites against obesity and biofouling. Mar. Drugs 2020, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.D.; Vitorino, I.; De la Cruz, M.; Díaz, C.; Cautain, B.; Annang, F.; Pérez-Moreno, G.; González Martínez, I.; Tormo, J.R.; Martín, J.M.; et al. Bioactivities and Extract Dereplication of Actinomycetales isolated from marine sponges. Front. Microbiol. 2019, 10, 727. [Google Scholar] [CrossRef] [PubMed]





| Oligonucleotide/Reference | Sequence (5′ to 3′) |
|---|---|
| ITS1/[18] | TCCGTAGGTGAACCTGCGG |
| ITS4/[18] | TCCTCCGCTTATTGATATGC |
| B49317/[19] | SCGAAATCGGTAGACGCTACG |
| A50272/[19] | ATTTGAACTGGTGACACGAG |
| rbcL-F/[20] | ATGTCACCACAAACAGAAACTAAAGC |
| rbcL-R/[20] | AATTCAAATTTAATTTCTTTCC |
| Standards | Calibration Curve | r2 | LOD (μg/mL) 1 | LOQ (μg/mL) 2 |
|---|---|---|---|---|
| Violaxanthin | y = 1,152,598,695x – 21,356 | 0.9997 | 0.3877 | 1.1748 |
| Lutein | y = 141,092,914x + 5527 | 0.9998 | 0.2867 | 0.8688 |
| ε-Carotene | y = 226,925,025x – 83,662 | 0.9996 | 2.3099 | 6.9998 |
| Chlorophyll-a | y = 7,471,178x + 2673 | 0.9998 | 1.4721 | 4.4608 |
| β-Carotene | y = 290,231,487x + 172,758 | 0.9997 | 1.9354 | 5.8649 |
| Molecular Marker | Length of Sequence (pb) | Query Cover (%) | Percentage of Identity (%) | GenBank Accession Number | IZTA Herbarium | Origin |
|---|---|---|---|---|---|---|
| ITS | 642 | 99 | 99.1 | OL451230/KM887387 | 45166 | Hidalgo/Delhi |
| trnL-trnF | 955 | 100 | 99.9 | OL456239/LC310979 | Hidalgo/Bangkok | |
| rbcL | 1266 | 100 | 100 | OL456230/LC214987 | Hidalgo/Bangkok | |
| ITS | 432 | 100 | 100 | OL451229/KR215626 | 45167 | Puebla/Delhi |
| trnL-trnF | 955 | 100 | 99.9 | OL456238/LC310979 | Puebla/Bangkok | |
| rbcL | 1266 | 100 | 100 | OL456234/LC214987 | Puebla/Bangkok | |
| ITS | 703 | 99 | 99.6 | OL451228/KM887387 | 45168 | Jalisco/Delhi |
| trnL-trnF | 955 | 100 | 99.7 | OL456237/LC310979 | Jalisco/Bangkok | |
| rbcL | 1266 | 100 | 100 | OL456233/LC214987 | Jalisco/Bangkok |
| Peak | Compound | RT (min) | BH | BE | BS | BX |
|---|---|---|---|---|---|---|
| 1 | ε-Carotene derivative | 5.6 | 0.072 ± 0.001 a | 0.061 ± 0.002 b | 0.052 ± ≤0.001 c | nd |
| 2 | Violaxanthin derivative | 6.0 | 0.012 ± ≤0.001 | 0.012 ± ≤0.001 | 0.012 ± ≤0.001 | 0.011 ± ≤0.001 |
| 3 | Violaxanthin derivative | 8.4 | 0.005 ± ≤0.001 | 0.004 ± ≤0.001 | 0.004 ± ≤0.001 | nd |
| 4 | Lutein | 12.2 | 0.921 ± 0.031 b | 0.988 ± 0.004 b | 0.969 ± 0.002 b | 1.149 ± 0.029 a |
| 5 | ε-Carotene derivative | 13.0 | 0.073 ± ≤0.001 b | 0.088 ± 0.002 a, b | 0.076 ± 0.006 a, b | 0.090 ± 0.003 a |
| 6 | ε-Carotene derivative | 13.4 | 0.070 ± 0.001 | 0.076 ± 0.001 | 0.073 ± 0.006 | 0.078 ± 0.002 |
| 7 | ε-Carotene derivative | 13.9 | 0.067 ± 0.001 | 0.068 ± 0.003 | 0.070 ± 0.004 | 0.072 ± ≤0.001 |
| 8 | Chlorophyll b derivative | 18.3 | 0.210 ± 0.019 b | 0.226 ± 0.001 b | 0.249 ± 0.007 b | 1.149 ± 0.068 a |
| 9 | Chlorophyll b derivative | 19.7 | 2.443 ± 0.2503 b | 1.724 ± 0.100 c | 1.342 ± 0.121 c | 6.759 ± 0.027 a |
| 10 | Chlorophyll b derivative | 20.0 | 0.197 ± 0.035 c | 0.376 ± 0.028 b | 0.343 ± 0.003 b | 0.862 ± 0.014 a |
| 11 | Chlorophyll b | 20.5 | 25.053 ± 0.939 a, b | 26.419 ± 0.026 a | 23.948 ± 0.223 a, b | 23.783 ± 0.798 b |
| 12 | Unidentified chlorophyll | 20.7 | nd | Nd | nd | 7.467 ± 0.064- |
| 13 | Unidentified chlorophyll | 21.3 | nd | 0.396 ± 0.056 c | 0.642 ± 0.060 b | 1.280 ± 0.009 a |
| 14 | Chlorophyll b derivative | 21.9 | 7.301 ± 0.466 a | 6.063 ± 0.203 b, c | 4.552 ± 0.124 c, d | 5.023 ± 0.109 c |
| 15 | Chlorophyll a | 23.1 | 0.522 ± 0.011 c | 0.566 ± 0.011 b, c | 1.097 ± 0.007 a | 0.630 ± 0.042 b |
| 16 | Unidentified chlorophyll | 26.3 | 1.393 ± 0.114 b | 2.064 ± 0.084 a | 1.275 ± 0.074 b | 1.535 ± 0.063 b |
| 17 | Unidentified chlorophyll | 26.9 | 0.546 ± 0.036 | 0.527 ± 0.035 | 0.374 ± 0.012 | 0.507 ± 0.074 |
| 18 | Unidentified chlorophyll | 28.8 | 2.307 ± 0.036 b | 2.440 ± 0.043 b | 2.076 ± 0.058 c | 2.642 ± 0.009 a |
| 19 | Unidentified chlorophyll | 29.4 | 0.365 ± 0.078 | 0.380 ± 0.042 | 0.309 ± 0.080 | 0.374 ± 0.027 |
| 20 | β-Carotene | 30.8 | 0.175 ± 0.007 b | 0.168 ± 0.002 b | 0.198 ± 0.006 a | 0.095 ± 0.003 c |
| 21 | 13-cis-Beta- Carotene | 31.4 | 0.004 ± 0.002 | 0.003 ± ≤0.001 | 0.007 ± 0.001 | nq |
| Total carotenoids | 1.575 ± 0.047 | 1.637 ± 0.005 | 1.662 ± 0.004 | 1.592 ± 0.038 | ||
| Total chlorophylls | 40.166 ± 1.906 b, c | 41.017 ± 0.060 b | 36.013 ± 0.055 c | 51.919 ± 0.863 a | ||
| Total pigments | 41.741 ± 1.953 b, c | 42.654 ± 0.054 b | 37.675 ± 0.051 c | 53.512 ± 0.826 a | ||
| Sample | TPC (µg GAE/mg Dry Extract) | DPPH• Scavenging (IC50, µg Dry Extract/mL) | NO Reduction in RAW 264.7 Cells (IC50, µg Dry Extract/mL) |
|---|---|---|---|
| BH | 67.6 ± 4.3 a | 130.6 ± 3.0 a | 181.7 ± 14.3 b |
| BE | 67.6 ± 5.6 a | 218.2 ± 6.0 c | 134.7 ± 3.8 a |
| BS | 70.3 ± 2.2 a | 249.9 ± 12.1 d | 122.4 ± 1.6 a |
| BX | 54.8 ± 5.8 b | 132.0 ± 17.8 b | 174.4 ± 7.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-García, M.; Garduño-Solórzano, G.; Lopes, G.; Sanchez, B.A.; Urbatzka, R.; Hentschke, G.S.; Campos, J.E.; Vasconcelos, V.M.O. Antioxidant, Anti-Inflammatory and Anti-Obesity Potential of Extracts Containing Phenols, Chlorophyll and Carotenoids from Mexican Wild Populations of Bacopa monnieri (L.) Wettst. Biology 2023, 12, 620. https://doi.org/10.3390/biology12040620
Martínez-García M, Garduño-Solórzano G, Lopes G, Sanchez BA, Urbatzka R, Hentschke GS, Campos JE, Vasconcelos VMO. Antioxidant, Anti-Inflammatory and Anti-Obesity Potential of Extracts Containing Phenols, Chlorophyll and Carotenoids from Mexican Wild Populations of Bacopa monnieri (L.) Wettst. Biology. 2023; 12(4):620. https://doi.org/10.3390/biology12040620
Chicago/Turabian StyleMartínez-García, Martha, Gloria Garduño-Solórzano, Graciliana Lopes, Begoña Astrain Sanchez, Ralph Urbatzka, Guilherme Scotta Hentschke, Jorge E. Campos, and Vitor Manuel Oliveira Vasconcelos. 2023. "Antioxidant, Anti-Inflammatory and Anti-Obesity Potential of Extracts Containing Phenols, Chlorophyll and Carotenoids from Mexican Wild Populations of Bacopa monnieri (L.) Wettst" Biology 12, no. 4: 620. https://doi.org/10.3390/biology12040620