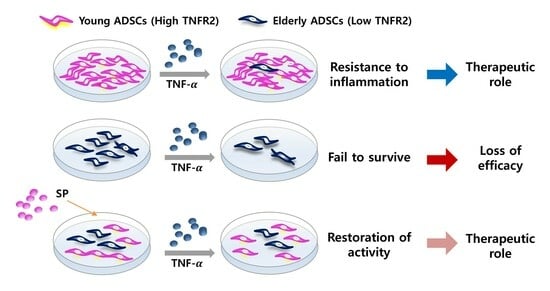
Preconditioning with Substance P Restores Therapeutic Efficacy of Aged ADSC by Elevating TNFR2 and Paracrine Potential
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Preparation of Cell Extracts and Western Blot Analysis
2.4. SP and TNF-α Treatment
2.5. WST-1 Assay
2.6. Cytokine Measurement
2.7. Tube Formation Assay
2.8. Statistical Analysis
3. Results
3.1. ADSC-E Is More Susceptible to Inflammatory Stress Than ADSC-Y
3.2. TNF-α Treatment Reduces TNFR2 Expression in ADSC-E, Impairing Repopulation and Paracrine Potentials
3.3. Pretreatment with SP Blocks the Loss of Cell Viability in ADSC-E When Exposed to TNF-α, and This Is Accompanied by an Increase in TNFR2 and Angiocrine Factors
3.4. Preconditioning with SP Sustains ADSC-E in a State of Active Akt Signaling, Rather Than Erk Signaling, in Response to TNF-α Treatment
3.5. Preconditioning with SP Enhance Paracrine Potential of ADSC-E under Inflammatory Condition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alicka, M.; Garbowska, K.K.; Kucharczyk, K.; Kepska, M.; Röcken, M.; Marycz, M. Age-dependent impairment of adipose-derived stem cells isolated from horses. Stem Cell Res. Ther. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Goodell, M.A.; Rando, T.A. Ageing and rejuvenation of tissue stem cells and their niches. Nat. Rev. Mol. Cell Biol. 2023, 24, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Piao, J.; Park, G.; Yoo, K.S.; Hong, H.S. Osteoporotic Conditions Influence the Activity of Adipose-Derived Stem Cells. Tissue Eng. Regen. Med. 2020, 17, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Butte, A.; Duscher, D.; Rennert, R.C.; Januszyk, M.; Anghel, E.; Maan, Z.N.; Whittam, A.J.; Perez, M.G.; Kosaraju, R.; Hu, M.S.; et al. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci. Rep. 2014, 4, 7144. [Google Scholar]
- Li, K.; Shi, G.; Lei, X.; Huang, Y.; Li, X.; Ba, L.; Qin, C. Age-related alteration in characteristics, function, and transcription features of ADSCs. Stem Cell Res. Ther. 2021, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Schipper, B.M.; Marra, K.G.; Zhang, W.; Donnenberg, A.D.; Rubin, J.P. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann. Plast. Surg. 2008, 60, 538–544. [Google Scholar] [CrossRef]
- Alt, E.U.; Senst, C.; Murthy, S.N.; Slakey, D.P.; Dupin, C.L.; Chaffin, A.E.; Kadowitz, P.J.; Izadpanah, R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012, 8, 215–225. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef]
- Sterodimas, A.; de Faria, J.; Nicaretta, B.; Pitanguy, I. Tissue engineering with adipose-derived stem cells (ADSCs): Current and future applications. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1886–1892. [Google Scholar] [CrossRef]
- Lee, T.J.; Shim, M.S.; Yu, T.; Choi, K.; Kim, D.I.; Lee, S.H.; Bhang, S.H. Bioreducible polymer micelles based on ac-id-degradable Poly(ethylene glycol)-poly(amino ketal) enhance the stromal cell-derived factor-1α gene transfection efficacy and therapeutic angiogenesis of human adipose-derived stem cells. Int. J. Mol. Sci. 2018, 19, 529. [Google Scholar] [CrossRef]
- Pan, J.; Alimujiang, M.; Chen, Q.; Shi, H.; Luo, X. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction−induced myocardial damage via downregulation of early growth response factor 1. J. Cell Biochem. 2019, 120, 4433–4443. [Google Scholar] [CrossRef]
- Li, M.; Ma, J.; Gao, Y.; Dong, M.; Zheng, Z.; Li, Y.; Tan, R.; She, Z.; Yang, L. Epithelial differentiation of human adi-pose-derived stem cells (hASCs) undergoing three-dimensional (3D) cultivation with collagen sponge scaffold (CSS) via an indirect co-culture strategy. Stem Cell Res. Ther. 2020, 11, 141. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, D.; Hong, H.S. Priming with a combination of FGF2 and HGF restores the impaired osteogenic differentiation of adipose-derived stem cells. Cells 2022, 11, 2042. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef]
- Mazini, L.; Ezzoubi, M.; Malka, G. Overview of current adipose-derived stem cell (ADSCs) processing involved in therapeutic advancements: Flow chart and regulation updates before and after COVID-19. Stem Cell Res. Ther. 2021, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Sun, J.; Zhao, Z.; Lei, W.; Chen, Y.; Wang, X.; Yang, J.; Shen, Z. A brief review: Adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res. Ther. 2017, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, J.; Brand, D.D.; Zheong, S.G. Role of TNF–TNF receptor 2 signal in regulatory T Cells and its therapeutic implications. Front. Immunol. 2018, 9, 784. [Google Scholar] [CrossRef]
- Chen, Z.; Palmer, T.D. Differential roles of TNFR1 and TNFR2 signaling in adult hippocampal neurogenesis. Brain. Behav. Immun. 2013, 30, 45–53. [Google Scholar] [CrossRef]
- Salomon, B.L.; Leclerc, M.; Tosello, J.; Ronin, E.; Piaggio, E.; Cohen, J.L. Tumor necrosis factor α and regulatory T Cells in oncoimmunology. Front. Immunol. 2018, 9, 444. [Google Scholar] [CrossRef]
- Joedicke, J.J.; Myers, L.; Carmody, A.B.; Messer, R.J.; Wajant, H.; Lang, K.S.; Lang, P.A.; Mak, T.W.; Hasenkrug, K.J.; Dittmer, U. Activated CD8+ T cells induce expansion of Vβ5+ regulatory T cells via TNFR2 signaling. J. Immunol. 2014, 193, 2952–2960. [Google Scholar] [CrossRef] [PubMed]
- Ghada, B.; Sheyda, B.; Lezin, C.; Barkestani, M.N.; Abdelgawad, M.E.; Uzan, G.; Naserian, S. TNFR2 is a crucial hub controlling mesenchymal stem cell biological and functional properties. Front. Cell Dev. Biol. 2020, 8, 596831. [Google Scholar]
- Dong, Y.; Fisher, R.; Naude, P.J.W.; Maier, O.; Nyakas, C.; Duffey, M.; Zee, E.A.V.D.; Dekens, D.; Douwenga, W.; Herrmann, A.; et al. Essential protective role of tumor necrosis factor receptor 2 in neurodegeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 12304–12309. [Google Scholar] [CrossRef]
- Sama, D.M.; Abdul, H.M.; Furman, J.L.; Artiushin, I.A.; Szymkowsk, D.E.; Scheff, S.W.; Norris, C.M. Inhibition of soluble tumor necrosis factor ameliorates synaptic alterations and Ca2+ dysregulation in aged rats. PLoS ONE 2012, 7, e38170. [Google Scholar] [CrossRef]
- Probert, L. TNF and its receptors in the CNS: The essential, the desirable and the deleterious effects. Neuroscience 2015, 302, 2–22. [Google Scholar] [CrossRef]
- Marchetti, L.; Klein, M.; Schlett, K.; Pfizenmaier, K.; Eisel, U. Tumor Necrosis Factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-Methyl-D-aspartate receptor activation. J. Biol. Chem. 2004, 279, 32869–32881. [Google Scholar] [CrossRef]
- Kelly, M.L.; Wang, M.; Crisostomo, P.R.; Abarbanell, A.M.; Herrmann, J.L.; Weil, B.R.; Meldrum, D.R. TNF Receptor 2, not TNF Receptor 1, enhances mesenchymal stem cell-mediated cardiac protection following acute ischemia. Shock 2010, 33, 602–607. [Google Scholar] [CrossRef]
- Yan, L.; Zheng, D.; Xu, R.-H. Critical role of tumor necrosisfactor signaling in mesenchymal stem cell-based therapy for autoimmune and inflammatory diseases. Front. Immunol. 2018, 9, 1658. [Google Scholar] [CrossRef]
- Densu, H.L.; Illiano, P.; Choi, J.S.; Ascorna, M.C.; Gao, H.; Lee, J.K.; Brambilla, R. TNFR2 signaling regulates the immunomodulatory function of oligodendrocyte precursor cells. Cells 2021, 19, 1785. [Google Scholar]
- Hong, H.; Lee, J.; Lee, E.; Kwon, Y.; Lee, E.; Ahn, W.; Jiang, M.; Kim, J.; Son, Y. A new role of substance P as an injury-inducible messenger for mobilization of CD29+ stromal-like cells. Nat. Med. 2009, 15, 425–435. [Google Scholar] [CrossRef]
- Lim, J.; Chung, E.; Son, Y. A neuropeptide, Substance-P, directly induces tissue-repairing M2 like macrophages by activating the PI3K/Akt/mTOR pathway even in the presence of IFNγ. Sci. Rep. 2017, 7, 9417. [Google Scholar] [CrossRef]
- Ahn, W.; Chi, G.; Kim, S.; Son, Y.; Zhang, M. Substance P reduces infarct size and mortality after ischemic stroke, possibly through the M2 polarization of microglia/macrophages and neuroprotection in the ischemic rat brain. Cell. Mol. Neurobiol. 2023, 43, 2035–2052. [Google Scholar] [CrossRef] [PubMed]
- Montana, G.; Lampiasi, N. Substance P induces HO-1 expression in RAW 264.7 cells promoting switch towards M2-Like macrophages. PLoS ONE 2016, 11, e0164720. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Park, J.S.; Kim, D.; Hong, H.S. Substance P hinders bile acid-induced hepatocellular injury by modulating oxidative stress and inflammation. Antioxidants 2022, 11, 920. [Google Scholar] [CrossRef]
- Kim, D.Y.; Piao, J.; Park, J.S.; Lee, D.; Hong, H.S. Substance P ameliorates TNF-α-mediated impairment of human aortic vascular cells in vitro. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1288–1297. [Google Scholar] [CrossRef]
- Baek, S.M.; Yu, S.Y.; Son, Y.; Hong, H.S. Substance P promotes the recovery of oxidative stress-damaged retinal pigmented epithelial cells by modulating Akt/GSK-3β signaling. Mol. Vis. 2016, 22, 1015–1023. [Google Scholar]
- Yu, J.; Nam, D.; Park, K.D. Substance P enhances cellular migration and inhibits senescence in human dermal fibroblasts under hyperglycemic conditions. Biochem. Biophys. Res. Commun. 2020, 522, 917–923. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, J.; Cho, H.; Park, J.H.; Yoo, K.H.; Jeong, I.; Hong, H.S. Preconditioning with Substance P Restores Therapeutic Efficacy of Aged ADSC by Elevating TNFR2 and Paracrine Potential. Biology 2023, 12, 1458. https://doi.org/10.3390/biology12121458
Piao J, Cho H, Park JH, Yoo KH, Jeong I, Hong HS. Preconditioning with Substance P Restores Therapeutic Efficacy of Aged ADSC by Elevating TNFR2 and Paracrine Potential. Biology. 2023; 12(12):1458. https://doi.org/10.3390/biology12121458
Chicago/Turabian StylePiao, Jiyuan, Hyunchan Cho, Jong Hyun Park, Ki Hyun Yoo, Ildoo Jeong, and Hyun Sook Hong. 2023. "Preconditioning with Substance P Restores Therapeutic Efficacy of Aged ADSC by Elevating TNFR2 and Paracrine Potential" Biology 12, no. 12: 1458. https://doi.org/10.3390/biology12121458