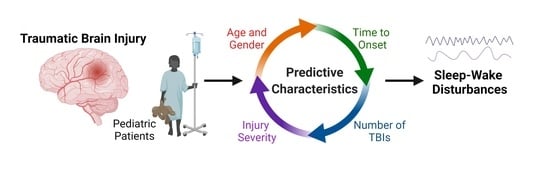
Traumatic Brain Injury Characteristics Predictive of Subsequent Sleep-Wake Disturbances in Pediatric Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Search Queries
2.2. Inclusion and Exclusion Criteria
2.3. Patient Data
2.4. Statistical Analyses
3. Results

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamani, A.; Mychasiuk, R.; Semple, B.D. Determinants of social behavior deficits and recovery after pediatric traumatic brain injury. Exp. Neurol. 2019, 314, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Ichkova, A.; Rodriguez-Grande, B.; Bar, C.; Villega, F.; Konsman, J.P.; Badaut, J. Vascular impairment as a pathological mechanism underlying long-lasting cognitive dysfunction after pediatric traumatic brain injury. Neurochem. Int. 2017, 111, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Keenan, H.T.; Presson, A.P.; Clark, A.E.; Cox, C.S.; Ewing-Cobbs, L. Longitudinal developmental outcomes after traumatic brain injury in young children: Are infants more vulnerable than toddlers? J. Neurotrauma 2018, 36, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Asarnow, R.F.; Newman, N.; Weiss, R.E.; Su, E. Association of attention-deficit/hyperactivity disorder diagnoses with pediatric traumatic brain injury: A meta-analysis. JAMA Pediatr. 2021, 175, 1009–1016. [Google Scholar] [CrossRef]
- Bloom, D.R.; Levin, H.S.; Ewing-Cobbs, L.; Saunders, A.E.; Song, J.; Fletcher, J.M.; Kowatch, R.A. Lifetime and novel psychiatric disorders after pediatric traumatic brain injury. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 572–579. [Google Scholar] [CrossRef]
- Acerini, C.L.; Tasker, R.C. Endocrine sequelae of traumatic brain injury in childhood. Horm. Res. Paediatr. 2007, 68 (Suppl. S5), 14–17. [Google Scholar] [CrossRef]
- Ortiz, J.B.; Sukhina, A.; Balkan, B.; Harootunian, G.; Adelson, P.D.; Lewis, K.S.; Oatman, O.; Subbian, V.; Rowe, R.K.; Lifshitz, J. Epidemiology of pediatric traumatic brain injury and hypothalamic-pituitary disorders in Arizona. Front. Neurol. 2020, 10, 1410. [Google Scholar] [CrossRef]
- Emelifeonwu, J.A.; Flower, H.; Loan, J.; McGivern, K.; Andrews, P.J. Prevalence of anterior pituitary dysfunction 12 months or more following traumatic brain injury in adults—A systematic review and meta-analysis. J. Neurotrauma 2019, 37, 217–226. [Google Scholar] [CrossRef]
- Gagner, C.; Landry-Roy, C.; Laine, F.; Beauchamp, M.H. Sleep-wake disturbances and fatigue after pediatric traumatic brain injury: A systematic review of the literature. J. Neurotrauma 2015, 32, 1539–1552. [Google Scholar] [CrossRef]
- Sandsmark, D.K.; Elliott, J.E.; Lim, M.M. Sleep-wake disturbances after traumatic brain injury: Synthesis of human and animal studies. Sleep 2017, 40, zsx044. [Google Scholar] [CrossRef] [Green Version]
- Suskauer, S.J.; Houtrow, A.J. Invited commentary on “The Report to Congress on the Management of Traumatic Brain Injury in Children”. Arch. Phys. Med. Rehabil. 2018, 99, 2389–2391. [Google Scholar] [CrossRef] [PubMed]
- Luther, M.; Poppert Cordts, K.M.; Williams, C.N. Sleep disturbances after pediatric traumatic brain injury: A systematic review of prevalence, risk factors, and association with recovery. Sleep 2020, 43, zsaa083. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.N.; Lim, M.M.; Shea, S.A. Sleep disturbance after pediatric traumatic brain injury: Critical knowledge gaps remain for the critically injured. Nat. Sci. Sleep 2018, 10, 225–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, S.R.; Alexander, J.; Moore, D.; Sasser, H.C.; Laurent, S.; King, J.; Bartel, S.; Callahan, B. Caregiver reports of common symptoms in children following a traumatic brain injury. NeuroRehabilitation 2004, 19, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Tham, S.W.; Fales, J.; Palermo, T.M. Subjective and objective assessment of sleep in adolescents with mild traumatic brain injury. J. Neurotrauma 2015, 32, 847–852. [Google Scholar] [CrossRef]
- Dan, B.; Boyd, S.G. A neurophysiological perspective on sleep and its maturation. Dev. Med. Child Neurol. 2006, 48, 773–779. [Google Scholar] [CrossRef]
- Volk, C.; Huber, R. Sleep to grow smart? Arch. Ital. Biol. 2015, 153, 99–109. [Google Scholar] [CrossRef]
- Ringli, M.; Huber, R. Developmental aspects of sleep slow waves: Linking sleep, brain maturation and behavior. Prog. Brain Res. 2011, 193, 63–82. [Google Scholar] [CrossRef]
- Timofeev, I.; Schoch, S.F.; LeBourgeois, M.K.; Huber, R.; Riedner, B.A.; Kurth, S. Spatio-temporal properties of sleep slow waves and implications for development. Curr. Opin. Physiol. 2020, 15, 172–182. [Google Scholar] [CrossRef]
- Lucke-Wold, B.P.; Smith, K.E.; Nguyen, L.; Turner, R.C.; Logsdon, A.F.; Jackson, G.J.; Huber, J.D.; Rosen, C.L.; Miller, D.B. Sleep disruption and the sequelae associated with traumatic brain injury. Neurosci. Biobehav. Rev. 2015, 55, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Fleming, M.K.; Smejka, T.; Henderson Slater, D.; van Gils, V.; Garratt, E.; Yilmaz Kara, E.; Johansen-Berg, H. Sleep disruption after brain injury is associated with worse motor outcomes and slower functional recovery. Neurorehabilit. Neural Repair 2020, 34, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Piantino, J.A.; Iliff, J.J.; Lim, M.M. The bidirectional link between sleep disturbances and traumatic brain injury symptoms: A role for glymphatic dysfunction? Biol. Psychiatry 2021, 5, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness: A practical scale. Lancet 1974, 304, 81–84. [Google Scholar] [CrossRef]
- Johnson, M.A.; Nishijima, D.K.; Kuppermann, N. The Association of Glasgow coma scale score with clinically important traumatic brain injuries in children. Pediatr. Emerg. Care 2020, 36, e610–e613. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, C.M.; Chisholm, K.H.; Madden, L.K.; Larsen, A.D.; De Longpré, T.; Stannard, D. Glasgow coma scale: Generating clinical standards. J. Neurosci. Nurs. 2019, 51, 142–146. [Google Scholar] [CrossRef]
- Jaffe, K.M.; Fay, G.C.; Polissar, N.L.; Martin, K.M.; Shurtleff, H.; Rivara, J.B.; Winn, H.R. Severity of pediatric traumatic brain injury and early neurobehavioral outcome: A cohort study. Arch. Phys. Med. Rehabil. 1992, 73, 540–547. [Google Scholar] [CrossRef]
- Babikian, T.; Asarnow, R. Neurocognitive outcomes and recovery after pediatric TBI: Meta-analytic review of the literature. Neuropsychology 2009, 23, 283–296. [Google Scholar] [CrossRef] [Green Version]
- Osorio, M.B.; Kurowski, B.G.; Beebe, D.; Taylor, H.G.; Brown, T.M.; Kirkwood, M.W.; Wade, S.L. Association of daytime somnolence with executive functioning in the first 6 months after adolescent traumatic brain injury. PM&R 2013, 5, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Theadom, A.; Starkey, N.; Jones, K.; Cropley, M.; Parmar, P.; Barker-Collo, S.; Feigin, V.L. Sleep difficulties and their impact on recovery following mild traumatic brain injury in children. Brain Inj. 2016, 30, 1243–1248. [Google Scholar] [CrossRef]
- Schwab, J.A. Multinomial Logistic Regression: Basic Relationships and Complete Problems; University of Texas: Austin, TX, USA, 2002. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- The R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Greene, W.H. Economic Analysis, 7th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2012. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-PLUS; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Cameron, A.C.; Trivedi, P.K. Regression Analysis of Count Data; Cambridge University Press: Cambridge, UK, 2013; Volume 53. [Google Scholar]
- Akaike, H. Maximum likelihood identification of Gaussian autoregressive moving average models. Biometrika 1973, 60, 255–265. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Spinger: New York, NY, USA, 2002. [Google Scholar]
- Arnold, T.W. Uninformative parameters and model selection using Akaike’s Information Criterion. J. Wildl. Manag. 2010, 74, 1175–1178. [Google Scholar] [CrossRef]
- Williams, C.N.; Hartman, M.E.; McEvoy, C.T.; Hall, T.A.; Lim, M.M.; Shea, S.A.; Luther, M.; Guilliams, K.P.; Guerriero, R.M.; Bosworth, C.C.; et al. Sleep-wake disturbances after acquired brain injury in children surviving critical care. Pediatr. Neurol. 2020, 103, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Yeo, V.; Phillips, N.L.; Bogdanov, S.; Brookes, N.; Epps, A.; Teng, A.; Naismith, S.L.; Lah, S. The persistence of sleep disturbance and its correlates in children with moderate to severe traumatic brain injury: A longitudinal study. Sleep Med. 2021, 81, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Tham, S.W.; Palermo, T.M.; Vavilala, M.S.; Wang, J.; Jaffe, K.M.; Koepsell, T.D.; Dorsch, A.; Temkin, N.; Durbin, D.; Rivara, F.P. The longitudinal course, risk factors, and impact of sleep disturbances in children with traumatic brain injury. J. Neurotrauma 2012, 29, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdanov, S.; Brookes, N.; Epps, A.; Naismith, S.L.; Teng, A.; Lah, S. Fatigue in children with moderate or severe traumatic brain injury compared with children with orthopedic injury: Characteristics and associated factors. J. Head Trauma Rehabil. 2021, 36, E108–E117. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Brookes, N.; Epps, A.; Naismith, S.L.; Teng, A.; Lah, S. Sleep disturbance in children with moderate or severe traumatic brain injury compared with children with orthopedic injury. J. Head Trauma Rehabil. 2019, 34, 122–131. [Google Scholar] [CrossRef]
- Vriend, J.; Corkum, P. Clinical management of behavioral insomnia of childhood. Psychol. Res. Behav. Manag. 2011, 4, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Donskoy, I.; Loghmanee, D. Insomnia in adolescence. Med. Sci. 2018, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- Calhoun, S.L.; Fernandez-Mendoza, J.; Vgontzas, A.N.; Liao, D.; Bixler, E.O. Prevalence of insomnia symptoms in a general population sample of young children and preadolescents: Gender effects. Sleep Med. 2014, 15, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, V.; Collop, N.A. Gender differences in sleep disorders. Curr. Opin. Pulm. Med. 2006, 12, 383–389. [Google Scholar] [CrossRef]
- Pavlovic, D.; Pekic, S.; Stojanovic, M.; Popovic, V. Traumatic brain injury: Neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary 2019, 22, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Riemann, L.; Zweckberger, K.; Unterberg, A.; El Damaty, A.; Younsi, A.; Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury Investigators and Participants. Injury causes and severity in pediatric traumatic brain injury patients admitted to the ward or intensive care unit: A collaborative European Neurotrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study. Front. Neurol. 2020, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Sayrs, L.W.; Ortiz, J.B.; Notrica, D.M.; Kirsch, L.; Kelly, C.; Stottlemyre, R.; Cohen, A.; Misra, S.; Green, T.R.; Adelson, P.D.; et al. Intimate partner violence, clinical indications, and other family risk factors associated with pediatric abusive head trauma. J. Interpers. Violence 2020. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.K.; Murphy, S.M.; Handmaker, H.; Lifshitz, J. Population-level epidemiology of concussion concurrent with domestic violence in Arizona, USA. J. Neurotrauma 2021, 38, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Notrica, D.M.; Kirsch, L.; Misra, S.; Kelly, C.; Greenberg, J.; Ortiz, J.B.; Rowe, R.K.; Lifshitz, J.; Adelson, P.D.; Stottlemyre, R.L.; et al. Evaluating abusive head trauma in children <5 years old: Risk factors and the importance of the social history. J. Pediatr. Surg. 2021, 56, 390–396. [Google Scholar] [CrossRef]
- Lind, K.; Toure, H.; Brugel, D.; Meyer, P.; Laurent-Vannier, A.; Chevignard, M. Extended follow-up of neurological, cognitive, behavioral and academic outcomes after severe abusive head trauma. Child Abuse Negl. 2016, 51, 358–367. [Google Scholar] [CrossRef]
- Broggi, M.; Ready, R.E. Academic skills, self-perceptions, and grades in university students with a history of multiple concussions: The mediating roles of processing speed and psychological symptoms. Clin. Neuropsychol. 2021. [Google Scholar] [CrossRef]
- Schatz, P.; Moser, R.S.; Covassin, T.; Karpf, R. Early indicators of enduring symptoms in high school athletes with multiple previous concussions. Neurosurgery 2011, 68, 1562–1567. [Google Scholar] [CrossRef] [Green Version]
- Masel, B.E.; DeWitt, D.S. Traumatic brain injury: A disease process, not an event. J. Neurotrauma 2010, 27, 1529–1540. [Google Scholar] [CrossRef] [Green Version]
- Corrigan, J.D.; Hammond, F.M. Traumatic brain injury as a chronic health condition. Arch. Phys. Med. Rehabil. 2013, 94, 1199–1201. [Google Scholar] [CrossRef]
- Thomasy, H.E.; Febinger, H.Y.; Ringgold, K.M.; Gemma, C.; Opp, M.R. Hypocretinergic and cholinergic contributions to sleep-wake disturbances in a mouse model of traumatic brain injury. Neurobiol. Sleep Circadian Rhythms 2017, 2, 71–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, R.K.; Striz, M.; Bachstetter, A.D.; Van Eldik, L.J.; Donohue, K.D.; O’Hara, B.F.; Lifshitz, J. Diffuse brain injury induces acute post-traumatic sleep. PLoS ONE 2014, 9, e82507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapp, Z.M.; Kumar, J.E.; Witcher, K.G.; Atluri, R.R.; Velasquez, J.A.; O’Neil, S.M.; Dziabis, J.E.; Bray, C.E.; Sheridan, J.F.; Godbout, J.P.; et al. Sleep disruption exacerbates and prolongs the inflammatory response to traumatic brain injury. J. Neurotrauma 2020, 37, 1829–1843. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.R.; Bassetti, C.L.; Valko, P.O.; Haybaeck, J.; Keller, M.; Clark, E.; Stocker, R.; Tolnay, M.; Scammell, T.E. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann. Neurol. 2009, 66, 555–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziebell, J.M.; Morganti-Kossmann, M.C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 2010, 7, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickstein, J.B.; Moldofsky, H. Sleep, cytokines and immune function. Sleep Med. Rev. 1999, 3, 219–228. [Google Scholar] [CrossRef]
- Krueger, J.M.; Majde, J.A.; Rector, D.M. Cytokines in immune function and sleep regulation. Handb. Clin. Neurol. 2011, 98, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Krueger, J.M.; Majde, J.A. Cytokines and sleep. Int. Arch. Allergy Imm. 1995, 106, 97–100. [Google Scholar] [CrossRef]
- Krueger, J.M.; Obal, F.; Fang, J.D.; Kubota, T.; Taishi, P. The role of cytokines in physiological sleep regulation. Ann. N. Y. Acad. Sci. 2001, 933, 211–221. [Google Scholar] [CrossRef]
- Krueger, J.M. The role of cytokines in sleep regulation. Curr. Pharm. Des. 2008, 14, 3408–3416. [Google Scholar] [CrossRef] [Green Version]
- Rowe, R.K.; Harrison, J.L.; Morrison, H.; Subbian, V.; Murphy, S.M.; Lifshitz, J. Acute post-traumatic sleep may define vulnerability to a second traumatic brain injury in mice. J. Neurotrauma 2018, 36, 1318–1334. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Zoumakis, M.; Papanicolaou, D.A.; Bixler, E.O.; Prolo, P.; Lin, H.M.; Vela-Bueno, A.; Kales, A.; Chrousos, G.P. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism 2002, 51, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.; Giordano, K.R.; Hur, Y.; Ortiz, J.B.; Morrison, H.; Godbout, J.P.; Murphy, S.M.; Lifshitz, J.; Rowe, R.K. Acute peripheral inflammation and post-traumatic sleep differ between sexes after experimental diffuse brain injury. Eur. J. Neurosci. 2019, 52, 2791–2814. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.G.; Boles, J.A.; Wagner, A.K. Chronic inflammation after severe traumatic brain injury: Characterization and associations with outcome at 6 and 12 months postinjury. J. Head Trauma Rehabil. 2015, 30, 369–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, V.A.; Catroppa, C.; Haritou, F.; Morse, S.; Rosenfeld, J.V. Identifying factors contributing to child and family outcome 30 months after traumatic brain injury in children. J. Neurol. Neurosurg. Psychiatry 2005, 76, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kovachy, B.; O’Hara, R.; Hawkins, N.; Gershon, A.; Primeau, M.M.; Madej, J.; Carrion, V. Sleep disturbance in pediatric PTSD: Current findings and future directions. J. Clin. Sleep Med. 2013, 9, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Charuvastra, A.; Cloitre, M. Safe enough to sleep: Sleep disruptions associated with trauma, posttraumatic stress, and anxiety in children and adolescents. Child Adolesc. Psychiatr. Clin. N. Am. 2009, 18, 877–891. [Google Scholar] [CrossRef]
- Mellman, T.A.; Hipolito, M.M. Sleep disturbances in the aftermath of trauma and posttraumatic stress disorder. CNS Spectr. 2006, 11, 611–615. [Google Scholar] [CrossRef]
- Hall Brown, T.S.; Belcher, H.M.E.; Accardo, J.; Minhas, R.; Briggs, E.C. Trauma exposure and sleep disturbance in a sample of youth from the National Child Traumatic Stress Network Core Data Set. Sleep Health 2016, 2, 123–128. [Google Scholar] [CrossRef]
- Simard, V.; Nielsen, T.A.; Tremblay, R.E.; Boivin, M.; Montplaisir, J.Y. Longitudinal study of preschool sleep disturbance: The predictive role of maladaptive parental behaviors, early sleep problems, and child/mother psychological factors. Arch. Pediatr. Adolesc. Med. 2008, 162, 360–367. [Google Scholar] [CrossRef]
- Pavlova, M.; Kopala-Sibley, D.C.; Nania, C.; Mychasiuk, R.; Christensen, J.; McPeak, A.; Tomfohr-Madsen, L.; Katz, J.; Palermo, T.M.; Noel, M. Sleep disturbance underlies the co-occurrence of trauma and pediatric chronic pain: A longitudinal examination. Pain 2020, 161, 821–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holley, A.L.; Wilson, A.C.; Noel, M.; Palermo, T.M. Post-traumatic stress symptoms in children and adolescents with chronic pain: A topical review of the literature and a proposed framework for future research. Eur. J. Pain 2016, 20, 1371–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Max, J.E.; Wilde, E.A.; Bigler, E.D.; MacLeod, M.; Vasquez, A.C.; Schmidt, A.T.; Chapman, S.B.; Hotz, G.; Yang, T.T.; Levin, H.S. Psychiatric disorders after pediatric traumatic brain injury: A prospective, longitudinal, controlled study. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.A.; Gamble, A.L. The role of sleep in childhood psychiatric disorders. Child Youth Care Forum 2009, 38, 327–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBourgeois, M.K.; Hale, L.; Chang, A.-M.; Akacem, L.D.; Montgomery-Downs, H.E.; Buxton, O.M. Digital media and sleep in childhood and adolescence. Pediatrics 2017, 140, S92–S96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McWood, L.M.; Zeringue, M.M.; Piñón, O.M.; Buckhalt, J.A.; El-Sheikh, M. Linear and nonlinear associations between the sleep environment, presleep conditions, and sleep in adolescence: Moderation by race and socioeconomic status. Sleep Med. 2021. [Google Scholar] [CrossRef]
- Tarokh, L.; Saletin, J.M.; Carskadon, M.A. Sleep in adolescence: Physiology, cognition and mental health. Neurosci. Biobehav. Rev. 2016, 70, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Campbell, I.G.; Grimm, K.J.; De Bie, E.; Feinberg, I. Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. Proc. Natl. Acad. Sci. USA 2012, 109, 5740–5743. [Google Scholar] [CrossRef] [Green Version]
- Pieters, S.; Van Der Vorst, H.; Burk, W.J.; Wiers, R.W.; Engels, R.C. Puberty-dependent sleep regulation and alcohol use in early adolescents. Alcohol. Clin. Exp. Res. 2010, 34, 1512–1518. [Google Scholar] [CrossRef]
- Wang, J.; Kwok, M.K.; Yeung, S.L.A.; Zhao, J.; Li, A.M.; Lam, H.S.; Leung, G.M.; Schooling, C.M. Age of puberty and sleep duration: Observational and Mendelian randomization study. Sci. Rep. 2020, 10, 3202. [Google Scholar] [CrossRef] [Green Version]
- Lucien, J.N.; Ortega, M.T.; Shaw, N.D. Sleep and puberty. Curr. Opin. Endocr. Metab. Res. 2021, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shaw, N.D.; Gill, S.; Lavoie, H.B.; Marsh, E.E.; Hall, J.E. Persistence of sleep-associated decrease in GnRH pulse frequency in the absence of gonadal steroids. J. Clin. Endocr. Metab. 2011, 96, 2590–2595. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.E.; Ram, N.; Susman, E.J.; Weinraub, M. Changes to sleep-wake behaviors are associated with trajectories of pubertal timing and tempo of secondary sex characteristics. J. Adolesc. 2018, 68, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Kalmbach, D.A.; Conroy, D.A.; Falk, H.; Rao, V.; Roy, D.; Peters, M.E.; Van Meter, T.E.; Korley, F.K. Poor sleep is linked to impeded recovery from traumatic brain injury. Sleep 2018, 41, zsy147. [Google Scholar] [CrossRef] [PubMed]



| Type of SWD | Males | Females | Total |
|---|---|---|---|
| circadian rhythm sleep disorder | 5.5% (6) | 3.1% (3) | 4.4% (9) |
| hypersomnia/excessive daytime sleepiness | 6.4% (7) | 9.2% (9) | 7.7% (16) |
| insomnia | 22.9% (25) | 28.6% (28) | 25.6% (53) |
| obstructive sleep apnea (OSA) | 10.1% (11) | 5.1% (5) | 7.7% (16) |
| sleep difficulties/sleep disorders | 39.5% (43) | 41.8% (41) | 40.6% (84) |
| other | 15.6% (17) | 12.2% (12) | 14.0% (29) |
| Model | Ka | Log-Lik b | AICc c | ΔAICc d | Wt e |
|---|---|---|---|---|---|
| Type ~ Age | 10 | −299.52 | 620.16 | 0.00 | 0.55 |
| Type ~ Age + Onset | 15 | −294.99 | 622.49 | 2.33 | 0.17 |
| Type ~ Age + TBIs | 15 | −295.09 | 622.68 | 2.52 | 0.16 |
| Type ~ Age + Onset + TBIs | 20 | −289.56 | 623.64 | 3.48 | 0.10 |
| Type ~ Age + Gender | 15 | −297.77 | 628.05 | 7.89 | 0.01 |
| Type ~ Age + Gender + TBIs | 20 | −293.24 | 630.99 | 10.83 | 0.00 |
| Type ~ Age + Gender + Onset | 20 | −293.42 | 631.35 | 11.19 | 0.00 |
| Type ~ Age + Severity | 20 | −293.58 | 631.68 | 11.52 | 0.00 |
| Type ~ Age + Gender + Onset + TBIs | 25 | −287.63 | 632.45 | 12.29 | 0.00 |
| Type ~ Age + Severity + Onset | 25 | −289.23 | 635.63 | 15.47 | 0.00 |
| Type ~ Age + Severity + TBIs | 25 | −290.17 | 637.53 | 17.37 | 0.00 |
| Type ~ Onset | 10 | −308.93 | 638.98 | 18.82 | 0.00 |
| Type ~ Onset + TBIs | 15 | −303.91 | 640.32 | 20.16 | 0.00 |
| Type ~ 1 | 5 | −315.11 | 640.52 | 20.36 | 0.00 |
| Type ~ Age + Severity + Onset + TBIs | 30 | −285.09 | 640.75 | 20.59 | 0.00 |
| Type ~ Age + Severity + Gender | 25 | −291.88 | 640.94 | 20.78 | 0.00 |
| Type ~ TBIs | 10 | −311.00 | 643.12 | 22.96 | 0.00 |
| Type ~ Age + Severity + Gender + Onset | 30 | −287.74 | 646.04 | 25.88 | 0.00 |
| Type ~ Gender + Onset | 15 | −307.14 | 646.80 | 26.64 | 0.00 |
| Type ~ Age + Severity + Gender + TBIs | 30 | −288.29 | 647.15 | 26.99 | 0.00 |
| Type ~ Gender | 10 | −313.07 | 647.26 | 27.10 | 0.00 |
| Type ~ Severity + Onset | 20 | −301.42 | 647.36 | 27.20 | 0.00 |
| Type ~ Severity | 15 | −307.61 | 647.74 | 27.58 | 0.00 |
| Type ~ Gender + Onset + TBIs | 20 | −301.78 | 648.08 | 27.92 | 0.00 |
| Type ~ Gender + TBIs | 15 | −308.90 | 650.31 | 30.15 | 0.00 |
| Type ~ Age + Severity + Gender + Onset + TBIs | 35 | −283.22 | 651.17 | 31.01 | 0.00 |
| Type ~ Severity + Onset + TBIs | 25 | −297.41 | 651.99 | 31.83 | 0.00 |
| Type ~ Severity + TBIs | 20 | −304.52 | 653.55 | 33.39 | 0.00 |
| Type ~ Severity + Gender | 20 | −305.70 | 655.91 | 35.75 | 0.00 |
| Type ~ Severity + Gender + Onset | 25 | −299.83 | 656.84 | 36.68 | 0.00 |
| Type ~ Severity + Gender + Onset + TBIs | 30 | −295.45 | 661.47 | 41.31 | 0.00 |
| Type ~ Severity + Gender + TBIs | 25 | −302.49 | 672.16 | 52.00 | 0.00 |
| Model | Ka | Log-Lik b | AICc c | ΔAICc d | Wt e |
|---|---|---|---|---|---|
| Onset ~ Age + Severity + TBIs | 6 | −492.15 | 996.70 | 0.00 | 0.50 |
| Onset ~ Age + Severity + Gender + TBIs | 7 | −491.92 | 998.37 | 1.67 | 0.22 |
| Onset ~ Age + TBIs | 4 | −495.33 | 998.83 | 2.13 | 0.17 |
| Onset ~ Age + Gender + TBIs | 5 | −495.15 | 1000.57 | 3.87 | 0.07 |
| Onset ~ Age | 3 | −498.61 | 1003.31 | 6.61 | 0.02 |
| Onset ~ Age + Gender | 4 | −498.12 | 1004.42 | 7.72 | 0.01 |
| Onset ~ Age + Severity | 5 | −498.07 | 1006.42 | 9.72 | 0.00 |
| Onset ~ Age + Severity + Gender | 6 | −497.54 | 1007.47 | 10.77 | 0.00 |
| Onset ~ TBIs | 3 | −502.17 | 1010.43 | 13.73 | 0.00 |
| Onset ~ Severity + TBIs | 5 | −500.77 | 1011.80 | 15.10 | 0.00 |
| Onset ~ Gender + TBIs | 4 | −501.99 | 1012.16 | 15.46 | 0.00 |
| Onset ~ Severity + Gender + TBIs | 6 | −500.55 | 1013.49 | 16.79 | 0.00 |
| Onset ~ 1 | 2 | −505.54 | 1015.11 | 18.41 | 0.00 |
| Onset ~ Gender | 3 | −505.07 | 1016.23 | 19.53 | 0.00 |
| Onset ~ Severity | 4 | −505.50 | 1019.16 | 22.46 | 0.00 |
| Onset ~ Severity + Gender | 5 | −505.01 | 1020.29 | 23.59 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerald, B.; Ortiz, J.B.; Green, T.R.F.; Brown, S.D.; Adelson, P.D.; Murphy, S.M.; Rowe, R.K. Traumatic Brain Injury Characteristics Predictive of Subsequent Sleep-Wake Disturbances in Pediatric Patients. Biology 2022, 11, 600. https://doi.org/10.3390/biology11040600
Gerald B, Ortiz JB, Green TRF, Brown SD, Adelson PD, Murphy SM, Rowe RK. Traumatic Brain Injury Characteristics Predictive of Subsequent Sleep-Wake Disturbances in Pediatric Patients. Biology. 2022; 11(4):600. https://doi.org/10.3390/biology11040600
Chicago/Turabian StyleGerald, Brittany, J. Bryce Ortiz, Tabitha R. F. Green, S. Danielle Brown, P. David Adelson, Sean M. Murphy, and Rachel K. Rowe. 2022. "Traumatic Brain Injury Characteristics Predictive of Subsequent Sleep-Wake Disturbances in Pediatric Patients" Biology 11, no. 4: 600. https://doi.org/10.3390/biology11040600