Spatial Dynamics and Fine-Scale Vertical Behaviour of Immature Eastern Australasian White Sharks (Carcharodon carcharias)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tag Deployment
2.2. Tag Details and Programming
2.3. Track Reconstruction
2.4. Depth and Temperature Data Processing
2.5. Statistical Analysis
3. Results
3.1. Deployment Summary
3.2. Horizontal Movements
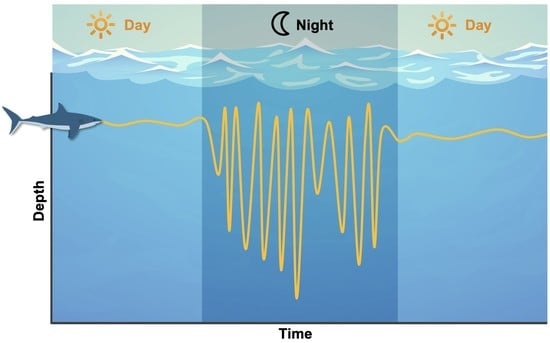
3.3. Vertical Activity
4. Discussion
4.1. Horizontal Movements
4.2. Diving Behaviour
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nathan, R.; Getz, W.M.; Revilla, E.; Holyoak, M.; Kadmon, R.; Saltz, D.; Smouse, P.E. A Movement Ecology Paradigm for Unifying Organismal Movement Research. Proc. Natl. Acad. Sci. USA 2008, 105, 19052–19059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeltsch, F.; Bonte, D.; Pe’er, G.; Reineking, B.; Leimgruber, P.; Balkenhol, N.; Schröder, B.; Buchmann, C.M.; Mueller, T.; Blaum, N.; et al. Integrating Movement Ecology with Biodiversity Research—Exploring New Avenues to Address Spatiotemporal Biodiversity Dynamics. Mov. Ecol. 2013, 1, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussey, N.E.; Kessel, S.T.; Aarestrup, K.; Cooke, S.J.; Cowley, P.D.; Fisk, A.T.; Harcourt, R.G.; Holland, K.N.; Iverson, S.J.; Kocik, J.F. Aquatic Animal Telemetry: A Panoramic Window into the Underwater World. Science 2015, 348, 1221–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boustany, A.M.; Davis, S.F.; Pyle, P.; Anderson, S.D.; Le Boeuf, B.J.; Block, B.A. Satellite Tagging: Expanded Niche for White Sharks. Nature 2002, 415, 35–36. [Google Scholar] [CrossRef] [PubMed]
- Bonfil, R.; Meÿer, M.; Scholl, M.C.; Johnson, R.; O’brien, S.; Oosthuizen, H.; Swanson, S.; Kotze, D.; Paterson, M. Transoceanic Migration, Spatial Dynamics, and Population Linkages of White Sharks. Science 2005, 310, 100–103. [Google Scholar] [CrossRef]
- Bruce, B.D.; Stevens, J.D.; Malcolm, H. Movements and Swimming Behaviour of White Sharks (Carcharodon carcharias) in Australian Waters. Mar. Biol. 2006, 150, 161–172. [Google Scholar] [CrossRef]
- Weng, K.C.; Boustany, A.M.; Pyle, P.; Anderson, S.D.; Brown, A.; Block, B.A. Migration and Habitat of White Sharks (Carcharodon carcharias) in the Eastern Pacific Ocean. Mar. Biol. 2007, 152, 877–894. [Google Scholar] [CrossRef]
- Nasby-Lucas, N.; Dewar, H.; Lam, C.H.; Goldman, K.J.; Domeier, M.L. White Shark Offshore Habitat: A Behavioral and Environmental Characterization of the Eastern Pacific Shared Offshore Foraging Area. PLoS ONE 2009, 4, e8163. [Google Scholar] [CrossRef] [Green Version]
- Carey, F.G.; Kanwisher, J.W.; Brazier, O.; Gabrielson, G.; Casey, J.G.; Pratt, H.L., Jr. Temperature and Activities of a White Shark, Carcharodon carcharias. Copeia 1982, 2, 254–260. [Google Scholar] [CrossRef]
- Skomal, G.B.; Braun, C.D.; Chisholm, J.H.; Thorrold, S.R. Movements of the White Shark Carcharodon carcharias in the North Atlantic Ocean. Mar. Ecol. Prog. Ser. 2017, 580, 1–16. [Google Scholar] [CrossRef]
- Winton, M.V. Fine-Scale Vertical Habitat Use of White Sharks at an Emerging Aggregation Site and Implications for Public Safety. Wildl. Res. 2021, 48, 345–360. [Google Scholar] [CrossRef]
- Franks, B.R.; Tyminski, J.P.; Hussey, N.E.; Braun, C.D.; Newton, A.L.; Thorrold, S.R.; Fischer, G.C.; Mcbride, B.; Hueter, R.E. Spatio-Temporal Variability in White Shark (Carcharodon carcharias) Movement Ecology During Residency and Migration Phases in the Western North Atlantic. Front. Mar. Sci. 2021, 8, 1630. [Google Scholar] [CrossRef]
- Huveneers, C.; Apps, K.; Becerril-García, E.E.; Bruce, B.; Butcher, P.A.; Carlisle, A.; Chapple, T.; Christiansen, H.; Cliff, G.; Curtis, T.; et al. Future Research Directions on the “Elusive” White Shark. Front. Mar. Sci. 2018, 5, 455. [Google Scholar] [CrossRef]
- Department of Sustainability, Environment, Water, Population and Communities. Recovery Plan for the White Shark (Carcharodon carcharias); Department of Sustainability, Environment, Water, Population and Communities: Canberra, Australia, 2013. Available online: https://www.awe.gov.au/environment/biodiversity/threatened/recovery-plans/recovery-plan-white-shark-carcharodon-carcharias (accessed on 20 February 2022).
- Rigby, C.L.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; Herman, K.J.; Jabado, R.W.; Liu, K.M.; Lowe, C.; et al. Carcharodon carcharias. The IUCN Red List of Threatened Species 2019: E.T3855A2878674. Available online: https://Dx.Doi.Org/10.2305/IUCN.UK.2019-3.RLTS.T3855A2878674.En (accessed on 6 October 2022).
- Spaet, J.L.Y. Carcharodon carcharias (Green Status Assessment). The IUCN Red List of Threatened Species 2021: E.T3855A385520221. Available online: https://www.iucnredlist.org/species/3855/212629880#green-assessment-information (accessed on 6 October 2022).
- Hillary, R.M.; Bravington, M.V.; Patterson, T.A.; Grewe, P.; Bradford, R.; Feutry, P.; Gunasekera, R.; Peddemors, V.; Werry, J.; Francis, M.P. Genetic Relatedness Reveals Total Population Size of White Sharks in Eastern Australia and New Zealand. Sci. Rep. 2018, 8, 2661. [Google Scholar] [CrossRef] [Green Version]
- Bruce, B.D.; Harasti, D.; Lee, K.; Gallen, C.; Bradford, R. Broad-Scale Movements of Juvenile White Sharks Carcharodon carcharias in Eastern Australia from Acoustic and Satellite Telemetry. Mar. Ecol. Prog. Ser. 2019, 619, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bonfil, R.; Francis, M.P.; Duffy, C.; Manning, M.J.; O’Brien, S. Large-Scale Tropical Movements and Diving Behavior of White Sharks Carcharodon carcharias Tagged off New Zealand. Aquat. Biol. 2010, 8, 115–123. [Google Scholar] [CrossRef]
- Duffy, C.A.J.; Francis, M.P.; Manning, M.J.; Bonfil, R. Regional Population Connectivity, Oceanic Habitat, and Return Migration Revealed by Satellite Tagging of White Sharks, Carcharodon carcharias, at New Zealand Aggregation Sites. In Global Perspectives on the Biology and Life History of the White Shark; Domeier, M.L., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 301–318. [Google Scholar]
- Bradford, R.; Patterson, T.A.; Rogers, P.J.; McAuley, R.; Mountford, S.; Huveneers, C.; Robbins, R.; Fox, A.; Bruce, B.D. Evidence of Diverse Movement Strategies and Habitat Use by White Sharks, Carcharodon carcharias, off Southern Australia. Mar. Biol. 2020, 167, 96. [Google Scholar] [CrossRef]
- Spaet, J.L.Y.; Patterson, T.A.; Bradford, R.W.; Butcher, P.A. Spatiotemporal Distribution Patterns of Immature Australasian White Sharks (Carcharodon carcharias). Sci. Rep. 2020, 10, 10169. [Google Scholar] [CrossRef]
- Spaet, J.L.Y.; Manica, A.; Brand, C.P.; Gallen, C.; Butcher, P.A. Environmental Conditions Are Poor Predictors of Immature White Shark Carcharodon carcharias Occurrences on Coastal Beaches of Eastern Australia. Mar. Ecol. Prog. Ser. 2020, 653, 167–179. [Google Scholar] [CrossRef]
- Bruce, B.D.; Bradford, R.W. Habitat Use and Spatial Dynamics of Juvenile White Sharks, Carcharodon carcharias, in Eastern Australia. In Global Perspectives on the Biology and Life History of the White Shark; Domeier, M.L., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 225–254. [Google Scholar]
- Coxon, J.L.; Butcher, P.A.; Spaet, J.L.Y.; Rizzari, J.R. Preliminary Data about Habitat Use of Subadult and Adult White Sharks (Carcharodon carcharias) in Eastern Australian Waters. Biology 2022, 11, 1443. [Google Scholar] [CrossRef]
- Francis, M.P.; Duffy, C.A.J.; Bonfil, R.; Manning, M.J. The Third Dimension: Vertical Habitat Use by White Sharks, Carcharodon carcharias. In Global Perspectives on the Biology and Life History of the White Shark; Domeier, M.L., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 319–342. [Google Scholar]
- Bradford, R.W.; Hobday, A.J.; Bruce, B.D. Identifying Juvenile White Shark Behaviour from Electronic Tag Data. In Global Perspectives on the Biology and Life History of the White Shark; Domeier, M.L., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 255–270. [Google Scholar]
- Jorgensen, S.J.; Arnoldi, N.S.; Estess, E.E.; Chapple, T.K.; Rückert, M.; Anderson, S.D.; Block, B.A. Eating or Meeting? Cluster Analysis Reveals Intricacies of White Shark (Carcharodon carcharias) Migration and Offshore Behavior. PLoS ONE 2012, 7, e47819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, S.J.; Reeb, C.A.; Chapple, T.K.; Anderson, S.; Perle, C.; Van Sommeran, S.R.; Fritz-Cope, C.; Brown, A.C.; Klimley, A.P.; Block, B.A. Philopatry and Migration of Pacific White Sharks. Proc. R. Soc. Lond. B Biol. Sci. 2010, 277, 679–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyomard, D.; Perry, C.; Tournoux, P.U.; Cliff, G.; Peddemors, V.; Jaquemet, S. An Innovative Fishing Gear to Enhance the Release of Non-Target Species in Coastal Shark-Control Programs: The SMART (Shark Management Alert in Real-Time) Drumline. Fish. Res. 2019, 216, 6–17. [Google Scholar] [CrossRef]
- Heupel, M.R.; Simpfendorfer, C.A.; Collins, A.B.; Tyminski, J.P. Residency and Movement Patterns of Bonnethead Sharks, Sphyrna Tiburo, in a Large Florida Estuary. Environ. Biol. Fishes 2006, 76, 47–67. [Google Scholar] [CrossRef]
- Lipscombe, R.S.; Spaet, J.L.Y.; Scott, A.; Lam, C.H.; Brand, C.P.; Butcher, P.A. Habitat Use and Movement Patterns of Tiger Sharks (Galeocerdo cuvier) in Eastern Australian Waters. ICES J. Mar. Sci. 2020, 77, 3127–3137. [Google Scholar] [CrossRef]
- Argos CLS. Argos User Manual Worldwide Tracking and Environmental Monitoring by Satellite. Ramonv. Saint-Anne CLS Serv. Argos. 2016. Available online: http://www.argos-system.org (accessed on 20 February 2022).
- Galuardi, B.; Lam, C.H.T. Telemetry Analysis of Highly Migratory Species. In Stock Identification Methods; Elsevier: Amsterdam, The Netherlands, 2014; pp. 447–476. [Google Scholar] [CrossRef]
- Bauer, R. RchivalTag: Analyzing Archival Tagging Data. R Package Version 0.1.2. Available online: https://CRAN.R-Project.Org/Package=RchivalTag2020 (accessed on 20 February 2022).
- R Core Team R: A Language and Environment for Statistical Computing (Version 4.0.5); R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 20 February 2022).
- Pante, E.; Simon-Bouhet, B. Marmap: A Package for Importing, Plotting and Analyzing Bathymetric and Topographic Data in R. PLoS ONE 2013, 8, e73051. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.D.; Robbins, W.D.; Peddemors, V.M. Decadal Trends in Shark Catches and Effort from the New South Wales, Australia, Shark Meshing Program 1950–2010. Mar. Freshw. Res. 2011, 62, 676–693. [Google Scholar] [CrossRef]
- Lee, K.; Butcher, P.; Harcourt, R.; Patterson, T.; Peddemors, V.; Roughan, M.; Harasti, D.; Smoothey, A.; Bradford, R. Oceanographic Conditions Associated with White Shark (Carcharodon carcharias) Habitat Use along Eastern Australia. Mar. Ecol. Prog. Ser. 2021, 659, 143–159. [Google Scholar] [CrossRef]
- Braun, C.D.; Galuardi, B.; Thorrold, S.R. HMMoce: An R Package for Improved Geolocation of Archival-Tagged Fishes Using a Hidden Markov Method. Methods Ecol. Evol. 2018, 9, 1212–1220. [Google Scholar] [CrossRef]
- Lutcavage, M.E.; Lam, C.H.; Galuardi, B. Seventeen Years and $3 Million Dollars Later: Performance of PSAT Tags Deployed on Atlantic Bluefin and Bigeye Tuna. Collect. Vol. Sci. Pap. ICCAT 2015, 71, 1176–1757. [Google Scholar]
- Lam, C.H.; Tam, C.; Kobayashi, D.R.; Lutcavage, M.E. Complex Dispersal of Adult Yellowfin Tuna from the Main Hawaiian Islands. Front. Mar. Sci. 2020, 7, 138. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, S.J.; Chapple, T.K.; Anderson, S.; Hoyos, M.; Reeb, C.; Block, B.A. Connectivity among White Shark Coastal Aggregation Areas in the Northeastern Pacific. In Global Perspectives on the Biology and Life History of the White Shark; Domeier, M.L., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 159–168. [Google Scholar]
- Nasby-Lucas, N.; Dewar, H.; Sosa-Nishizaki, O.; Wilson, C.; Hyde, J.R.; Vetter, R.D.; Wraith, J.; Block, B.A.; Kinney, M.J.; Sippel, T. Movements of Electronically Tagged Shortfin Mako Sharks (Isurus oxyrinchus) in the Eastern North Pacific Ocean. Anim. Biotelemetry 2019, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Dewar, H.; Domeier, M.; Nasby-Lucas, N. Insights into Young of the Year White Shark, Carcharodon Carcharias, Behavior in the Southern California Bight. J. Appl. Phycol. 2004, 70, 133–143. [Google Scholar] [CrossRef]
- Shaw, R.L.; Curtis, T.H.; Metzger, G.; Mccallister, M.P.; Newton, A.; Fischer, G.C.; Ajemian, M.J.; Wells, D. Three-Dimensional Movements and Habitat Selection of Young White Sharks (Carcharodon carcharias) across a Temperate Continental Shelf Ecosystem. Front. Mar. Sci. 2021, 8, 643831. [Google Scholar] [CrossRef]
- Andrzejaczek, S.; Gleiss, A.C.; Pattiaratchi, C.B.; Meekan, M.G. Patterns and Drivers of Vertical Movements of the Large Fishes of the Epipelagic. Rev. Fish Biol. Fish. 2019, 29, 335–354. [Google Scholar] [CrossRef]
- Weng, K.C.; O’Sullivan, J.B.; Lowe, C.G.; Winkler, C.E.; Dewar, H.; Block, B.A. Movements, Behavior and Habitat Preferences of Juvenile White Sharks Carcharodon Carcharias in the Eastern Pacific. Mar. Ecol. Prog. Ser. 2007, 338, 211–224. [Google Scholar] [CrossRef]
- Nakamura, I.; Watanabe, Y.Y.; Papastamatiou, Y.P.; Sato, K.; Meyer, C.G. Yo-Yo Vertical Movements Suggest a Foraging Strategy for Tiger Sharks Galeocerdo Cuvier. Mar. Ecol. Prog. Ser. 2011, 424, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Papastamatiou, Y.P.; Iosilevskii, G.; Leos-Barajas, V.; Brooks, E.J.; Howey, L.A.; Chapman, D.D.; Watanabe, Y.Y. Optimal Swimming Strategies and Behavioral Plasticity of Oceanic Whitetip Sharks. Sci. Rep. 2018, 8, 551. [Google Scholar] [CrossRef] [Green Version]
- Andrzejaczek, S.; Gleiss, A.C.; Lear, K.O.; Pattiaratchi, C.B.; Chapple, T.K.; Meekan, M.G. Biologging Tags Reveal Links between Fine-Scale Horizontal and Vertical Movement Behaviors in Tiger Sharks (Galeocerdo cuvier). Front. Mar. Sci. 2019, 6, 229. [Google Scholar] [CrossRef]
- Grainger, R.; Peddemors, V.M.; Raubenheimer, D.; Machovsky-Capuska, G.E. Diet Composition and Nutritional Niche Breadth Variability in Juvenile White Sharks (Carcharodon carcharias). Front. Mar. Sci. 2020, 7, 422. [Google Scholar] [CrossRef]
- Stevenson, M.L.; Hanchet, S.M. Inshore Trawl Survey of the West Coast of the South Island and Tasman and Golden Bays, March—April 2009 (KAH0904). In New Zealand Fisheries Assessment Report; Ministry of Fisheries Wellington: Wellington, New Zealand, 2009; Volume 4, pp. 1–69. Available online: https://docs.niwa.co.nz/library/public/FAR2010-11.pdf (accessed on 20 February 2022).
- Young, J.W.; Hobday, A.J.; Campbell, R.A.; Kloser, R.J.; Bonham, P.I.; Clementson, L.A.; Lansdell, M.J. The Biological Oceanography of the East Australian Current and Surrounding Waters in Relation to Tuna and Billfish Catches off Eastern Australia. Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 720–733. [Google Scholar] [CrossRef]
- Goldman, K.J.; Anderson, S.D. Space Utilization and Swimming Depth of White Sharks, Carcharodon carcharias, at the South Farallon Islands, Central California. Environ. Biol. Fishes 1999, 56, 351–364. [Google Scholar] [CrossRef]
- Holland, K.N.; Wetherbee, B.M.; Lowe, C.G.; Meyer, C.G. Movements of Tiger Sharks (Galeocerdo cuvier) in Coastal Hawaiian Waters. Mar. Biol. 1999, 134, 665–673. [Google Scholar] [CrossRef]
- Domeier, M.L.; Nasby-Lucas, N. Migration Patterns of White Sharks Carcharodon carcharias Tagged at Guadalupe Island, Mexico, and Identification of an Eastern Pacific Shared Offshore Foraging Area. Mar. Ecol. Prog. Ser. 2008, 370, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.L.; Tinker, M.T.; Estes, J.A.; Koch, P.L. Ontogenetic and Among-Individual Variation in Foraging Strategies of Northeast Pacific White Sharks Based on Stable Isotope Analysis. PLoS ONE 2012, 7, e45068. [Google Scholar] [CrossRef] [Green Version]
- Hussey, N.E.; Mccann, H.M.; Cliff, G.; Dudley, S.F.J.; Wintner, S.P.; Fisk, A.T. Size-Based Analysis of Diet and Trophic Position of the White Shark (Carcharodon carcharias) in South African Waters. In Global Perspectives on the Biology and Life History of the White Shark; CRC Press: Boca Raton, FL, USA, 2012; pp. 27–50. [Google Scholar]
- Braun, C.D.; Arostegui, M.C.; Thorrold, S.R.; Papastamatiou, Y.P.; Gaube, P.; Fontes, J.; Afonso, P. The Functional and Ecological Significance of Deep Diving by Large Marine Predators. Ann. Rev. Mar. Sci. 2021, 14, 1–31. [Google Scholar] [CrossRef]
- Spaet, J.L.Y.; Lam, C.H.; Braun, C.D.; Berumen, M.L. Extensive Use of Mesopelagic Waters by a Scalloped Hammerhead Shark (Sphyrna lewini) in the Red Sea. Anim. Biotelemetry 2017, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Gaube, P.; Braun, C.D.; Lawson, G.L.; McGillicuddy, D.J.; Della Penna, A.; Skomal, G.B.; Fischer, C.; Thorrold, S.R. Mesoscale Eddies Influence the Movements of Mature Female White Sharks in the Gulf Stream and Sargasso Sea. Sci. Rep. 2018, 8, 7363. [Google Scholar] [CrossRef] [Green Version]
- Larsen, T.; Ventura, M.; Andersen, N.; O’Brien, D.M.; Piatkowski, U.; McCarthy, M.D. Tracing Carbon Sources through Aquatic and Terrestrial Food Webs Using Amino Acid Stable Isotope Fingerprinting. PLoS ONE 2013, 8, e73441. [Google Scholar] [CrossRef] [Green Version]
- Le Croizier, G.; Lorrain, A.; Sonke, J.E.; Hoyos-Padilla, E.M.; Galván-Magaña, F.; Santana-Morales, O.; Aquino-Baleytó, M.; Becerril-García, E.E.; Muntaner-López, G.; Ketchum, J. The Twilight Zone as a Major Foraging Habitat and Mercury Source for the Great White Shark. Environ. Sci. Technol. 2020, 54, 15872–15882. [Google Scholar] [CrossRef]
- Larsen, T.; Yokoyama, Y.; Fernandes, R. Radiocarbon in Ecology: Insights and Perspectives from Aquatic and Terrestrial Studies. Methods Ecol. Evol. 2018, 9, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Horton, T.W.; Hauser, N.; Zerbini, A.N.; Francis, M.P.; Domeier, M.L.; Andriolo, A.; Costa, D.P.; Robinson, P.W.; Duffy, C.A.J.; Nasby-Lucas, N. Route Fidelity during Marine Megafauna Migration. Front. Mar. Sci. 2017, 4, 422. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, K.C.; Miller, S.P.; Huestis, S.P.; Spiess, F.N. Three-dimensional Modeling of a Magnetic Reversal Boundary from Inversion of Deep-tow Measurements. J. Geophys. Res. Solid Earth 1980, 85, 3670–3680. [Google Scholar] [CrossRef]
- Meyer, C.G.; Holland, K.N.; Papastamatiou, Y.P. Sharks Can Detect Changes in the Geomagnetic Field. J. R. Soc. Interface 2005, 2, 129–130. [Google Scholar] [CrossRef] [Green Version]
- Klimley, A.P. Highly Directional Swimming by Scalloped Hammerhead Sharks, Sphyrna Lewini, and Subsurface Irradiance, Temperature, Bathymetry, and Geomagnetic Field. Mar. Biol. 1993, 117, 1–22. [Google Scholar] [CrossRef]
- Lohmann, K.J.; Lohmann, C.M.F.; Putman, N.F. Magnetic Maps in Animals: Nature’s GPS. J. Exp. Biol. 2007, 210, 3697–3705. [Google Scholar] [CrossRef] [Green Version]
- Lawson, G.L.; Castleton, M.R.; Block, B.A. Movements and Diving Behavior of Atlantic Bluefin Tuna Thunnus Thynnus in Relation to Water Column Structure in the Northwestern Atlantic. Mar. Ecol. Prog. Ser. 2015, 400, 245–265. [Google Scholar] [CrossRef] [Green Version]
- Gunn, J.; Block, B. Advances in Acoustic, Archival, and Satellite Tagging of Tunas. Fish Physiol. 2001, 19, 167–224. [Google Scholar] [CrossRef]
- Wilson, S.G.; Lutcavage, M.E.; Brill, R.W.; Genovese, M.P.; Cooper, A.B.; Everly, A.W. Movements of Bluefin Tuna (Thunnus Thynnus) in the Northwestern Atlantic Ocean Recorded by Pop-up Satellite Archival Tags. Mar. Biol. 2005, 146, 409–423. [Google Scholar] [CrossRef]
- Sepulveda, C.A.; Kohin, S.; Chan, C.; Vetter, R.; Graham, J.B. Movement Patterns, Depth Preferences, and Stomach Temperatures of Free-Swimming Juvenile Mako Sharks, Isurus Oxyrinchus, in the Southern California Bight. Mar. Biol. 2004, 145, 191–199. [Google Scholar] [CrossRef]
- Rohner, C.A.; Bealey, R.; Fulanda, B.M.; Prebble, C.E.M.; Williams, S.M.; Pierce, S.J. Vertical Habitat Use by Black and Striped Marlin in the Western Indian Ocean. Mar. Ecol. Prog. Ser. 2022, 690, 165–183. [Google Scholar] [CrossRef]
- Buckley, T.W.; Miller, B.S. Feeding Habits of Yellowfin Tuna Associated with Fish Aggregation Devices in American Samoa. Bull. Mar. Sci. 1994, 55, 445–459. [Google Scholar]
- Scott, M.D.; Cattanach, K.L. Diel Patterns in Aggregations of Pelagic Dolphins and Tunas in the Eastern Pacific. Mar. Mammal Sci. 1998, 14, 401–422. [Google Scholar] [CrossRef]
- Jorgensen, S.J.; Gleiss, A.C.; Kanive, P.E.; Chapple, T.K.; Anderson, S.D.; Ezcurra, J.M.; Brandt, W.T.; Block, B.A. In the Belly of the Beast: Resolving Stomach Tag Data to Link Temperature, Acceleration and Feeding in White Sharks (Carcharodon carcharias). Anim. Biotelemetry 2015, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Heithaus, M.R. The Biology of Tiger Sharks, Galeocerdo Cuvier, in Shark Bay, Western Australia: Sex Ratio, Size Distribution, Diet, and Seasonal Changes in Catch Rates. Environ. Biol. Fishes 2001, 61, 25–36. [Google Scholar] [CrossRef]
- Afonso, A.S.; Hazin, F.H.V. Vertical Movement Patterns and Ontogenetic Niche Expansion in the Tiger Shark, Galeocerdo Cuvier. PLoS ONE 2015, 10, e0116720. [Google Scholar] [CrossRef]
- Andrzejaczek, S.; Lucas, T.C.; Goodman, M.C.; Hussey, N.E.; Armstrong, A.J.; Carlisle, A.; Coffey, D.M.; Gleiss, A.C.; Huveneers, C.; Jacoby, D.M.P.; et al. Diving into the vertical dimension of elasmobranch movement ecology. Sci. Adv. 2022, 8, eabo1754. [Google Scholar] [CrossRef]




| Shark ID | PSAT MiniPat Serial # | SLRT [Spot-6] Serial # | Acoustic Serial # | FL [cm] | Sex | Release Date | Release Latitude [° S] | Release Longitude [° E] | Pop Off Latitude [° S] | Pop Off Longitude [° E] | Pop Off Date | First Detection | Last Detection | Time at Liberty [d] | Depth Max. [m] | Temp Min. [°C] | Temp Max. [°C] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SLRT | Acoustic | SLRT | Acoustic | ||||||||||||||||
| 60 | 16P2399 | 16U0795 | 1241668 | 320 | F | 2018-11-16 | −28.84 | 153.59 | − | - | - | ND | 2016-12-01 | ND | 2018-01-23 | 298 [-] | - | - | - |
| 106 | 16P2195 | 16U2270 | 1241647 | 247 | F | 2017-08-17 | −32.16 | 152.52 | − | - | - | 2017-08-14 | 2017-06-21 | 2018-02-26 | 2017-11-04 | 193 [-] | - | - | - |
| 160 | 16P2288 | 16U2391 | 1268708 | 235 | M | 2017-08-17 | −32.18 | 152.55 | − | - | - | 2017-08-17 | ND | 2019-02-14 | ND | 546 [-] | - | - | - |
| 179 | 16P2362 | 16U2771 | 1268684 | 250 | M | 2017-09-13 | −29.49 | 153.37 | − | - | - | 2017-11-17 | 2017-10-09 | 2019-06-18 | 2017-10-09 | 644 [-] | - | - | - |
| 180 | 16P2232 | 12S1390 | 1241628 | 210 | F | 2017-09-12 | −29.45 | 153.37 | −33.53 | 151.37 | 2018-02-02 | 2017-09-12 | 2019-10-19 | 2018-01-08 | 2020-02-15 | 886 [140] | 136 | 14.7 | 24.8 |
| 227 | 16P2397 | 12S1393 | 1268704 | 269 | M | 2017-10-24 | −32.17 | 152.52 | −33.91 | 151.51 | 2018-01-25 | 2017-10-24 | 2017-11-24 | 2018-03-22 | 2019-07-12 | 595 [93] | 188 | 13.1 | 23.4 |
| 229 | 16P2400 | 16U2392 | 1268678 | 224 | M | 2017-10-25 | −29.11 | 153.45 | − | - | - | 2017-11-01 | 2017-12-03 | 2018-09-05 | 2017-12-03 | 316 [-] | - | - | - |
| 234 | 16P2189 | 16U2387 | 1268683 | 277 | M | 2017-10-28 | −28.89 | 153.59 | −35.13 | 150.87 | 2018-04-26 | 2017-11-20 | 2018-08-22 | 2019-04-18 | 2019-10-14 | 726 [180] | 600 | 8.6 | 28.9 |
| 251 * | 16P2398 | 12S1391 | 1268705 | 224 | M | 2017-11-15 | −31.83 | 152.75 | −32.77 | 152.24 | 2017-12-28 | 2017-11-15 | 2017-12-25 | 2018-01-10 | 2018-03-31 | 137 [-] | - | - | - |
| 290 | 16P2203 | 16U2390 | 1241625 | 252 | M | 2018-07-27 | −30.33 | 153.14 | − | - | - | 2018-09-18 | 2018-09-18 | 2019-02-09 | 2018-09-18 | 197 [-] | - | - | - |
| 315 | 16P2314 | 16U2706 | 1268672 | 176 | M | 2018-08-29 | −29.10 | 153.45 | − | - | - | 2018-08-29 | 2018-08-16 | 2019-07-30 | 2019-08-16 | 352 [-] | - | - | - |
| 338 | 16P2186 | n/a | 1277240 | 236 | F | 2018-11-11 | −29.08 | 153.45 | − | - | - | n/a | 2019-08-18 | n/a | 2019-08-18 | 337 [-] | - | - | - |
| 341 | 16P2248 | 16U2393 | 1268671 | 252 | F | 2018-12-04 | −31.81 | 152.75 | − | - | - | 2018-12-04 | 2018-12-12 | 2019-06-09 | 2020-01-31 | 423 [-] | - | - | - |
| 343 | 16P2204 | n/a | 1268695 | 175 | M | 2018-12-04 | −32.16 | 152.51 | − | - | - | n/a | 2019-05-03 | n/a | 2020-05-20 | 533 [-] | - | - | - |
| 345 | 16P2190 | n/a | 1241614 | 202 | F | 2018-12-05 | −32.16 | 152.51 | − | - | - | n/a | 2018-12-29 | n/a | 2020-02-19 | 441 [-] | - | - | - |
| 354 | 18P1880 | n/a | 1304664 | 244 | F | 2019-05-30 | −29.10 | 153.45 | −37.84 | 148.46 | 2019-10-22 | n/a | 2019-07-03 | n/a | 2019-08-25 | 146 [146] | 520 | 9.6 | 23.5 |
| 355 | 18P1877 | n/a | 1304663 | 213 | F | 2019-06-11 | −29.11 | 153.46 | −38.38 | 147.97 | 2019-12-07 | n/a | 2019-08-25 | n/a | 2019-08-25 | 180 [180] | 128 | 14.2 | 22.5 |
| 356 | 18P1950 | n/a | 1304668 | 184 | F | 2019-06-16 | −28.83 | 153.61 | −41.23 | 148.70 | 2019-12-13 | n/a | 2019-06-16 | n/a | 2019-11-03 | 140 [180] | 424 | 10.8 | 22.9 |
| 357 | 18P1881 | n/a | 1304673 | 219 | M | 2019-07-09 | −29.08 | 153.45 | −39.20 | 148.43 | 2020-01-05 | n/a | 2019-08-13 | n/a | 2020-06-19 | 346 [180] | 632 | 8.1 | 21.6 |
| 358 | 18P1486 | 16U2769 | 1304630 | 260 | F | 2019-07-11 | −28.79 | 153.61 | − | - | - | 2018-04-19 | 2019-10-14 | 2020-05-27 | 2019-10-18 | 322 [-] | - | - | - |
| 359 | 18P1485 | 16U1461 | 1304633 | 186 | F | 2019-07-11 | −28.88 | 153.61 | −38.74 | 147.69 | 2020-02-10 | 2018-12-03 | 2019-09-19 | 2020-05-19 | 2019-09-19 | 313 [180] | 79.5 | 19.0 | 21.5 |
| 360 ** | 18P1879 | n/a | 1304674 | 230 | F | 2019-07-12 | −29.09 | 153.44 | −47.68 | 122.13 | - | n/a | 2019-09-29 | n/a | 2019-10-03 | 84 [-] | - | - | - |
| 364 | 18P1876 | 19U0148 | 1268692 | 177 | F | 2019-07-18 | −32.16 | 152.52 | − | - | - | 2019-07-18 | 2019-07-30 | 2020-06-26 | 2019-08-25 | 345 [-] | - | - | - |
| 365 | 18P1878 | 16U2701 | 1241627 | 174 | M | 2019-07-18 | −32.16 | 152.51 | −32.17 | 152.52 | 2019-12-11 | 2019-11-07 | 2019-07-28 | 2019-09-04 | 2020-03-06 | 232 [146] | 116 | 14.2 | 29.3 |
| 366 | 18P1951 | 16U2704 | 1268679 | 193 | M | 2019-07-18 | −32.17 | 152.52 | −37.05 | 150.13 | 2020-01-16 | 2019-07-18 | 2019-08-08 | 2019-11-07 | 2020-03-11 | 238 [180] | 528 | 9.9 | 23.6 |
| 367 | 18P1873 | 16U2516 | 1268679 | 218 | M | 2019-07-18 | −32.16 | 152.51 | −38.04 | 147.64 | 2020-01-14 | 2019-07-08 | 2019-08-11 | 2019-08-20 | 2020-06-19 | 337 [180] | 144 | 12.8 | 20.6 |
| 375 | 18P1875 | 16U2388 | 1304649 | 227 | F | 2019-07-27 | −28.86 | 153.61 | −42.05 | 152.77 | 2020-03-01 | 2019-07-31 | 2019-10-12 | 2019-12-30 | 2019-12-19 | 156 [180] | 568 | 7.8 | 24.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spaet, J.L.Y.; Butcher, P.A.; Manica, A.; Lam, C.H. Spatial Dynamics and Fine-Scale Vertical Behaviour of Immature Eastern Australasian White Sharks (Carcharodon carcharias). Biology 2022, 11, 1689. https://doi.org/10.3390/biology11121689
Spaet JLY, Butcher PA, Manica A, Lam CH. Spatial Dynamics and Fine-Scale Vertical Behaviour of Immature Eastern Australasian White Sharks (Carcharodon carcharias). Biology. 2022; 11(12):1689. https://doi.org/10.3390/biology11121689
Chicago/Turabian StyleSpaet, Julia L. Y., Paul A. Butcher, Andrea Manica, and Chi Hin Lam. 2022. "Spatial Dynamics and Fine-Scale Vertical Behaviour of Immature Eastern Australasian White Sharks (Carcharodon carcharias)" Biology 11, no. 12: 1689. https://doi.org/10.3390/biology11121689