Assessment of Composite with Fibers as a Support for Antibacterial Nanomaterials: A Case Study of Bacterial Cellulose, Polylactide and Usual Textile
Abstract
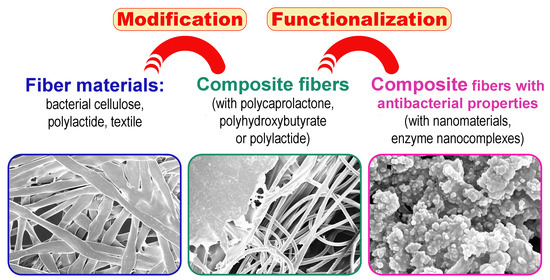
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Functionalization of Composite Materials
2.3. Estimation of Antibacterial Activity of Modified Fibers
2.4. Enzymatic Activity of Composit Fibrous Materials
3. Results
3.1. Structure of Composite Fiber Materials with PCL and PHB
3.2. Funcional Characteristics of Composite Fiber Materials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, M.K.; Mirica, K.A. Self-Organized Frameworks on Textiles (SOFT): Conductive Fabrics for Simultaneous Sensing, Capture, and Filtration of Gases. J. Am. Chem. Soc. 2017, 139, 16759–16767. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Khan, S.B.; Kamal, T.; Anwar, Y.; Alamry, K.A.; Asiri, A.M. Anti-bacterial chitosan/zinc phthalocyanine fibers supported metallic and bimetallic nanoparticles for the removal of organic pollutants. Carbohydr. Polym. 2017, 173, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Selvasudha, N.; Sweety, J.P.; Dhanalekshmi, U.M.; Devi, N.S.D. Smart antimicrobial textiles for healthcare professionals and individuals. In Antimicrobial Textiles from Natural Resources, 1st ed.; Mondal, I.H., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 455–484. [Google Scholar] [CrossRef]
- Singh, A.; Lee, Y.; Dressick, W.J. Self-Cleaning Fabrics for Decontamination of Organophosphorous Pesticides and Related Chemical Agents. Adv. Mater. 2004, 16, 2112–2115. [Google Scholar] [CrossRef]
- Conte, M.P.; Lau, K.H.; Ulijn, R.V. Biocatalytic Self-Assembly Using Reversible and Irreversible Enzyme Immobilization. ACS Appl. Mater. Interfaces 2017, 9, 3266–3271. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.E.; Senecal, K.J.; Goddard, J.M. Immobilization of chymotrypsin on hierarchical nylon 6,6 nanofiber improves enzyme performance. Colloids Surf. B Biointerfaces 2017, 154, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Efremenko, E.N.; Lyagin, I.V.; Klyachko, N.L.; Bronich, T.; Zavyalova, N.V.; Jiang, Y.; Kabanov, A.V. A simple and highly effective catalytic nanozyme scavenger for organophosphorus neurotoxins. J. Control. Release 2017, 247, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Lyagin, I.V.; Efremenko, E.N. Biomolecular engineering of biocatalysts hydrolyzing neurotoxic organophosphates. Biochimie 2018, 144, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Aslanli, A.; Lyagin, I.; Efremenko, E. Novel approach to Quorum Quenching: Rational design of antibacterials in combination with hexahistidine-tagged organophosphorus hydrolase. Biol. Chem. 2018, 399, 869–879. [Google Scholar] [CrossRef]
- Aslanli, A.; Lyagin, I.; Efremenko, E. Charges’ interaction in polyelectrolyte (nano)complexing of His6-OPH with peptides: Unpredictable results due to imperfect or useless concept? Int. J. Biol. Macromol. 2019, 140, 368–376. [Google Scholar] [CrossRef]
- Kim, M.; Gkikas, M.; Huang, A.; Kang, J.W.; Suthiwangcharoen, N.; Nagarajan, R.; Olsen, B.D. Enhanced activity and stability of organophosphorus hydrolase via interaction with an amphiphilic polymer. Chem. Commun. (Camb) 2014, 50, 5345–5348. [Google Scholar] [CrossRef] [Green Version]
- vander Straeten, A.; Bratek-Skicki, A.; Germain, L.; D’Haese, C.; Eloy, P.; Fustin, C.A.; Dupont-Gillain, C. Protein-polyelectrolyte complexes to improve the biological activity of proteins in layer-by-layer assemblies. Nanoscale 2017, 9, 17186–17192. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Robatjazi, S.M.; Sadri, M.; Mosaabadi, J.M. Immobilization of organophosphorus hydrolase enzyme by covalent attachment on modified cellulose microfibers using different chemical activation strategies: Characterization and stability studies. Chin. J. Chem. Eng. 2019, 27, 191–199. [Google Scholar] [CrossRef]
- Lyagin, I.; Stepanov, N.; Frolov, G.; Efremenko, E. Combined Modification of Fiber Materials by Enzymes and Metal Nanoparticles for Chemical and Biological Protection. Int. J. Mol. Sci. 2022, 23, 1359. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Prudnikova, S.V.; Kiselev, E.G.; Nemtsev, I.V.; Vasiliev, A.D.; Kuzmin, A.P.; Shishatskaya, E.I. Bacterial cellulose (BC) and BC composites: Production and properties. Nanomaterials 2022, 12, 192. [Google Scholar] [CrossRef]
- Xu, Y.; Li, S.; Yue, X.; Lu, W. Review of silver nanoparticles (AgNPs)-cellulose antibacterial composites. BioRes 2018, 13, 2150–2170. [Google Scholar] [CrossRef]
- Aslanli, A.; Lyagin, I.; Stepanov, N.; Presnov, D.; Efremenko, E. Bacterial Cellulose Containing Combinations of Antimicrobial Peptides with Various QQ Enzymes as a Prototype of an “Enhanced Antibacterial” Dressing: In Silico and In Vitro Data. Pharmaceutics 2020, 12, 1155. [Google Scholar] [CrossRef]
- Aslanli, A.; Stepanov, N.; Razheva, T.; Podorozhko, E.A.; Lyagin, I.; Lozinsky, V.I.; Efremenko, E.N. Enzymatically functionalized composite materials based on nanocellulose and poly(vinylalcohol) cryogel and possessing antimicrobial activity. Materials 2019, 12, 3619. [Google Scholar] [CrossRef] [Green Version]
- Bhuiyan, M.A.R.; Wang, L.; Shaid, A.; Shanks, R.A.; Ding, J. Advances and applications of chemical protective clothing system. J. Ind. Text. 2019, 49, 97–138. [Google Scholar] [CrossRef]
- Li, T.T.; Sun, L.; Zhong, Y.; Peng, H.K.; Ren, H.T.; Zhang, Y.; Lin, J.-H.; Lou, C.-W. Silk fibroin/polycaprolactone-polyvinyl alcohol directional moisture transport composite film loaded with antibacterial drug-loading microspheres for wound dressing materials. Int. J. Biol. Macromol. 2022, 207, 580–591. [Google Scholar] [CrossRef]
- Sabapathy, P.C.; Devaraj, S.; Anburajan, P.; Parvez, A.; Kathirvel, P.; Qi, X. Active polyhydroxybutyrate (PHB)/sugarcane bagasse fiber-based anti-microbial green composite: Material characterization and degradation studies. Appl. Nanosci. 2021; in press. [Google Scholar] [CrossRef]
- Makarov, I.; Vinogradov, M.; Gromovykh, T.; Lutsenko, S.; Feldman, N.; Shambilova, G.; Sadykova, V. Antifungal Composite Fibers Based on Cellulose and Betulin. Fibers 2018, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Pušnik Črešnar, K.; Aulova, A.; Bikiaris, D.N.; Lambropoulou, D.; Kuzmič, K.; Fras Zemljič, L. Incorporation of metal-based nanoadditives into the PLA matrix: Effect of surface properties on antibacterial activity and mechanical performance of PLA nanoadditive films. Molecules 2021, 26, 4161. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.; Moreira, I.P.; Gomes, F.; Cunha, F.; Henriques, M.; Fangueiro, R. Recent Trends in Protective Textiles against Biological Threats: A Focus on Biological Warfare Agents. Polymers 2022, 14, 1599. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, J.; Han, S.; Jiao, T.; Bai, Z.; Zhou, J.; Zhang, L.; Peng, Q. Preparation of palladium nanoparticles decorated polyethyleneimine/polycaprolactone composite fibers constructed by electrospinning with highly efficient and recyclable catalytic performances. Catalysts 2019, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.S.; Kim, H.K.; Ghim, M.S.; Hong, M.W.; Kim, Y.Y.; Cho, Y.S. Evaluation of the antibacterial activity and cell response for 3D-printed polycaprolactone/nanohydroxyapatite scaffold with zinc oxide coating. Polymers 2020, 12, 2193. [Google Scholar] [CrossRef]
- Frolov, G.; Lyagin, I.; Senko, O.; Stepanov, N.; Pogorelsky, I.; Efremenko, E. Metal nanoparticles for improving bactericide functionality of usual fibers. Nanomaterials 2020, 10, 1724. [Google Scholar] [CrossRef]
- Efremenko, E.; Votchitceva, Y.; Aliev, T.; Varfolomeev, S. Recombinant Plasmid DNA pTes-His-oph and Producer of Oligohistidine-Containing Organophosphate Hydrolase. RU Patent 2255975C1, 10 July 2005. Available online: https://patents.google.com/patent/RU2255975C1 (accessed on 23 August 2022).
- Efremenko, E.; Votchitseva, Y.; Plieva, F.; Galaev, I.; Mattiasson, B. Purification of His6-organophosphate hydrolase using monolithic supermacroporous polyacrylamide cryogels developed for immobilized metal affinity chromatography. Appl. Microbiol. Biotechnol. 2006, 70, 558–563. [Google Scholar] [CrossRef]
- Votchitseva, Y.A.; Efremenko, E.N.; Aliev, T.K.; Varfolomeyev, S.D. Properties of hexahistidine-tagged organophosphate hydrolase. Biochemistry 2006, 71, 167–172. [Google Scholar] [CrossRef]
- Ismayilov, I.T.; Stepanov, N.A.; Efremenko, E.N.; Abbasov, V.M. Evaluation of biocidal properties of vegetable oil-based corrosion inhibitors using bioluminescent enzymatic method. Mosc. Univ. Chem. Bull. 2015, 70, 197–201. [Google Scholar] [CrossRef]
- Stepanov, N.; Senko, O.; Perminova, I.; Efremenko, E. A new approach to assess the effect of various humic compounds on the metabolic activity of cells participating in methanogenesis. Sustainability 2019, 11, 3158. [Google Scholar] [CrossRef] [Green Version]
- Bychuk, M.A. Preparation and Properties of Polymer Films Based on Poly-3-Hydroxybutyrate and Poly-ɛ-Caprolactone. Ph.D Dissertation, Moscow State University of Design and Technology, Moscow, Russia, 2016. [Google Scholar]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Li, J.; Chen, M.; Zhang, S.; He, R.; Wang, N. Environmentally friendly and antimicrobial bilayer structured fabrics with integrated interception and sterilization for personal protective mask. Separat. Purificat. Technol. 2022, 294, 121165. [Google Scholar] [CrossRef]
- Sirotkina, M.; Efremenko, E.N. Rhodococcus lactonase with organophosphate hydrolase (OPH) activity and His6-tagged OPH with lactonase activity: Evolutionary proximity of the enzymes and new possibilities in their application. Appl. Microbiol. Biotechnol. 2014, 98, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeev, S.D.; Efremenko, E.N. (Eds.) Organophosphorus Neurotoxins, 1st ed.; RIOR: Moscow, Russia, 2020; 379p. [Google Scholar] [CrossRef]
- Lyagin, I.; Efremenko, E. Enzymes, Reacting with Organophosphorus Compounds as Detoxifiers: Diversity and Functions. Int. J. Mol. Sci. 2021, 22, 1761. [Google Scholar] [CrossRef]
- Lyagin, I.; Efremenko, E. Enzymes for detoxification of various mycotoxins: Origins and mechanisms of catalytic action. Molecules 2019, 24, 2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyagin, I.; Maslova, O.; Stepanov, N.; Presnov, D.; Efremenko, E. Assessment of Composite with Fibers as a Support for Antibacterial Nanomaterials: A Case Study of Bacterial Cellulose, Polylactide and Usual Textile. Fibers 2022, 10, 70. https://doi.org/10.3390/fib10090070
Lyagin I, Maslova O, Stepanov N, Presnov D, Efremenko E. Assessment of Composite with Fibers as a Support for Antibacterial Nanomaterials: A Case Study of Bacterial Cellulose, Polylactide and Usual Textile. Fibers. 2022; 10(9):70. https://doi.org/10.3390/fib10090070
Chicago/Turabian StyleLyagin, Ilya, Olga Maslova, Nikolay Stepanov, Denis Presnov, and Elena Efremenko. 2022. "Assessment of Composite with Fibers as a Support for Antibacterial Nanomaterials: A Case Study of Bacterial Cellulose, Polylactide and Usual Textile" Fibers 10, no. 9: 70. https://doi.org/10.3390/fib10090070