Effect of Pulse Current Mode on Microstructure, Composition and Corrosion Performance of the Coatings Produced by Plasma Electrolytic Oxidation on AZ31 Mg Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
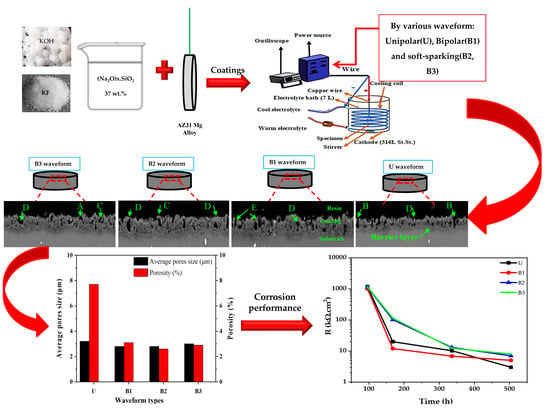
2.2. Plasma Electrolytic Oxidation Treatment
2.3. Coatings Characterization
2.4. Evaluation of Corrosion Behavior of the Coatings
3. Results and Discussion
3.1. Cell Current Density-Time Responses during PEO Treatment
3.2. Surface Morphology of the Coatings
3.3. Cross-Sectional Observation, Structure and Composition of the Coatings
3.4. Long-Term EIS Measurements
4. Conclusions
- The cell current densities were dependent on the waveform. Two reduction steps for stage III in current density response were observed during the PEO treatment of Mg alloy using the bipolar and soft-sparking waveforms. By the second step, a non-uniform distribution of discharges was happened. The time that the second step appeared was depending on the waveform as it happened sooner at higher cathodic duty cycle.
- Surface morphology of the coatings was net-like and/or scaffold containing a micro-pores network, micro-cracks and granule of oxide compounds. Applying the various waveforms had no noticeable effect on the surface morphology.
- Deep pores were originated by B- and D-type of discharges in the coating produced by unipolar waveform. By using the bipolar ones, D- and E-types of discharges were promoted which shifted the pores toward the outer layer of coatings. Increasing the cathodic duty cycle (soft-sparking waveforms), eliminated even the pores produced by E-type, providing the lowest porosity in the coatings.
- Corrosion performance of the PEO coatings was mostly determined by their inner layer resistances. This is because of the higher compactness achieved for this layer because of the formation of MgF2 phase.
- After 4 days of immersion in 3.5 wt. % NaCl solution, the inner layer resistances were almost the same for all coatings. However, the coatings produced by unipolar and usual bipolar waveforms showed sharp decays in inner layer resistances after 1 week of exposure, while a minor reduction was observed for the coatings produced by soft-sparking waveforms, which means that they are more protective due to their lower porosity. After 3 weeks, the barrier effect of outer layer was lost for the unipolar-produced coating, while it is preserved for others. The presence of an inductive loop for all coatings indicated that the substrate is under local corrosion attack at this time. However, both coatings produced using the soft-sparking waveforms provided the highest inner layer resistances (i.e., the highest corrosion performance).
Author Contributions
Funding
Conflicts of Interest
References
- Rogov, A.B.; Yerokhin, A.; Matthews, A. The role of cathodic current in plasma electrolytic oxidation of aluminum: Phenomenological concepts of the “soft sparking” mode. Langmuir 2017, 33, 11059–11069. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.S.; Chou, C.C. Review of the Soft Sparking Issues in Plasma Electrolytic Oxidation. Metals 2018, 8, 105. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O. Improving the performance of magnesium alloys for automotive applications. WIT Trans. Built. Environ. 2014, 137, 531–544. [Google Scholar]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef]
- Tjiang, F.; Ye, L.W.; Huang, Y.J.; Chou, C.C.; Tsai, D.S. Effect of processing parameters on soft regime behavior of plasma electrolytic oxidation of Magnesium. Ceram. Int. 2017, 43, 567–572. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O.; Nie, X. Processing Microstructure Relationships in the Plasma Electrolytic Oxidation (PEO) Coating of a Magnesium Alloy. Mater. Sci. Appl. 2014, 5, 124–139. [Google Scholar] [CrossRef]
- Yao, Z.; Xu, Y.; Jiang, Z.; Wang, F. Effects of cathode pulse at low frequency on the structure and composition of plasma electrolytic oxidation ceramic coatings. J. Alloys Compd. 2009, 488, 273–278. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Hashimoto, T.; Skeldon, P.; Thompson, G.E. Characterization of AC PEO coatings on magnesium alloys. Surf. Coat. Technol. 2009, 203, 2207–2220. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O.; Su, J.F.; Nie, X. A study of the interactive effects of hybrid current modes on the tribological properties of a PEO ( plasma electrolytic oxidation ) coated AM60B Mg-alloy. Surf. Coat. Technol. 2013, 215, 421–430. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O.; Nie, X. The influence of pulse timing and current mode on the microstructure and corrosion behaviour of a plasma electrolytic oxidation (PEO) coated AM60B magnesium alloy. J. Alloys Compd. 2012, 541, 41–48. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 48, 105–203. [Google Scholar] [CrossRef]
- Martin, J.; Melhem, A.; Shchedrina, I.; Duchanoy, T.; Nominé, A.; Henrion, G.; Czerwiec, T.; Belmonte, T. Effects of electrical parameters on plasma electrolytic oxidation of aluminium. Surf. Coat. Technol. 2013, 221, 70–76. [Google Scholar] [CrossRef]
- Gebarowski, W.; Pietrzyk, S. Influence of the Cathodic Pulse on the Formation and Morphology of Oxide Coating on Aluminium Produced by Plasma Electrolytic Oxidation. Arch. Metall Mater. 2013, 58, 241–245. [Google Scholar] [CrossRef]
- Hussein, R.O.; Zhang, P.; Nie, X.; Xia, Y.; Northwood, D.O. The effect of current mode and discharge type on the corrosion resistance of plasma electrolytic oxidation (PEO) coated magnesium alloy AJ62. Surf. Coat. Technol. 2011, 206, 1990–1997. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Guo, B.; Tian, J.; Liu, H.; Zhou, J.; Liu, W.; Xu, T. Effects of NaAlO2 on structure and corrosion resistance of microarc oxidation coatings formed on AM60B magnesium alloy in phosphate-KOH electrolyte. Surf. Coat. Technol. 2005, 199, 121–126. [Google Scholar] [CrossRef]
- Gao, Y.; Yerokhin, A.; Matthews, A. Effect of current mode on PEO treatment of magnesium in Ca- and P-containing electrolyte and resulting coatings. Appl. Sur. Sci. 2014, 316, 558–568. [Google Scholar] [CrossRef]
- Pan, Y.K.; Chen, C.Z.; Wang, D.G.; Zhao, T.G. Improvement of corrosion and biological properties of micro arc oxidized coatings on Mg-Zn-Zr alloy by optimizing negative power density parameters. Colloids Surf. B Biointerfaces 2014, 113, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Chang, L. Growth regularity of ceramic coating on magnesium alloy by plasma electrolytic oxidation. J. Alloys Compd. 2009, 468, 462–465. [Google Scholar] [CrossRef]
- Han, B.; Yang, Y.; Deng, H.; Chen, Y.; Yang, C. Plasma-electrolytic-oxidation coating containing Y2O3 nanoparticles on AZ91 magnesium alloy. Int. J. Electrochem. Sci. 2018, 13, 5681–5697. [Google Scholar] [CrossRef]
- Han, B.; Yang, Y.; Li, J.; Deng, H.; Yang, C. Effects of the graphene additive on the corrosion resistance of the plasma electrolytic oxidation (PEO) coating on the AZ91 magnesium alloy. Int. J. Electrochem. Sci. 2018, 13, 9166–9182. [Google Scholar] [CrossRef]
- Dong, H.; Blawert, C.; Srinivasan, P.B. Plasma electrolytic oxidation treatment of magnesium alloys. In Surface Engineering of Light Alloys; Woodhead, P.: New York, NY, USA, 2010; pp. 155–183. [Google Scholar]
- Kumar, M.A.; Kwon, S.H.; Jung, H.C.; Shin, K.S. Corrosion protection performance of single and dual Plasma Electrolytic Oxidation (PEO) coating for aerospace applications. Mater. Chem. Phys. 2015, 149, 480–486. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Santamaria, M.; Asghari, M. Effects of pulse current mode on plasma electrolytic oxidation of 7075 Al in Na2WO4 containing solution: From unipolar to soft-sparking regime. Electrochim. Acta 2018, 284, 618–629. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Golozar, M.A.; Lu, X.; Blawert, C.; Zheludkevich, M.L. The effect of pulse waveforms on surface morphology, composition and corrosion behavior of Al2O3 and Al2O3/TiO2 nano-composite PEO coatings on 7075 aluminum alloy. Surf. Coat. Technol. 2017, 324, 208–221. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, M.; Yang, X.; Huang, P.; Xu, K. Effect of Na2SiO3 solution concentration of micro-arc oxidation process on lap-shear strength of adhesive-bonded magnesium alloys. Appl. Surf. Sci. 2014, 314, 447–452. [Google Scholar] [CrossRef]
- Duan, H.; Yan, C.; Wang, F. Growth process of plasma electrolytic oxidation films formed on magnesium alloy AZ91D in silicate solution. Electrochim. Acta 2007, 52, 5002–5009. [Google Scholar] [CrossRef]
- Timoshenko, A.V.; Magurova, Y.V. Application of oxide coatings to metals in electrolyte solutions by microplasma methods. Rev. Met. Madrid. 2000, 36, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Tian, B.; Du, K.; Zhang, H.; Wang, F. Preparation and Corrosion Performance of PEO Coating With Low Porosity on Magnesium Alloy AZ91D In Acidic KF System. Int. J. Electrochem. Sci. 2011, 6, 5228–5248. [Google Scholar]
- Mann, R.; Hansal, W.E.G.; Hansal, S. Effects of pulsed current on plasma electrolytic oxidation. Trans. IMF 2014, 92, 297–304. [Google Scholar] [CrossRef]
- Aliramezani, R.; Raeissi, K.; Santamaria, M.; Hakimizad, A. Effects of Pulse Current Mode on Plasma Electrolytic Oxidation of 7075 Al in KMnO4 Containing Solution. J. Electrochem. Soc. 2017, 164, 690–698. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Xue, Z.G.; Wang, Q.; Wu, X.Q.; Matykina, E.; Skeldon, P.; Thompson, G.E. New findings on properties of plasma electrolytic oxidation Coatings from study of an Al-Cu-Li alloy. Electrochim. Acta 2013, 107, 358–378. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloy. 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Influence of process parameters on electrolytic plasma discharging behaviour and aluminum oxide coating microstructure. Surf. Coat. Technol. 2010, 205, 1659–1667. [Google Scholar] [CrossRef]
- Jin, F.; Chu, P.K.; Xu, G.; Zhao, J.; Tang, D.; Tong, H. Structure and mechanical properties of magnesium alloy treated by micro-arc discharge oxidation using direct current and high-frequency bipolar pulsing modes. Mater. Sci. Eng. A 2006, 436, 123–126. [Google Scholar] [CrossRef]
- Jaspard-mécuson, F.; Czerwiec, T.; Henrion, G.; Belmonte, T. Tailored aluminium oxide layers by bipolar current adjustment in the Plasma Electrolytic Oxidation (PEO) process. Surf. Coat. Technol. 2007, 201, 8677–8682. [Google Scholar] [CrossRef]
- Liu, F.; Yu, J.; Song, Y.; Shan, D.; Han, E.H. Effect of potassium fluoride on the in-situ sealing pores of plasma electrolytic oxidation film on AM50 Mg alloy. Mater. Chem. Phys. 2015, 162, 452–460. [Google Scholar] [CrossRef]
- Santamaria, M.; Di Quarto, F.; Zanna, S.; Marcus, P. The influence of surface treatment on the anodizing of magnesium in alkaline solution. Electrochim. Acta 2011, 56, 10533–10542. [Google Scholar] [CrossRef]
- Habazaki, H.; Fushimi, K.; Shimizu, K.; Skeldon, P.; Thompson, G.E. Fast migration of fluoride ions in growing anodic titanium oxide. Electrochem. Commun. 2007, 9, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Kazanski, B.; Kossenko, A.; Zinigrad, M.; Lugovskoy, A. Applied Surface Science Fluoride ions as modifiers of the oxide layer produced by plasma electrolytic oxidation on AZ91D magnesium alloy. Appl. Sur. Sci. 2013, 287, 461–466. [Google Scholar] [CrossRef]
- Curioni, M.; Salamone, L.; Scenini, F.; Santamaria, M.; Di Natale, M. A mathematical description accounting for the superfluous hydrogen evolution and the inductive behaviour observed during electrochemical measurements on magnesium. Electrochim. Acta 2018, 274, 343–352. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Shin, S.H.; Hussain, I.; Koo, B.H. Investigation of Hybrid PEO Coatings on AZ31B Magnesium Alloy in Alkaline K2ZrF6–Na2SiO3 Electrolyte Solution. Prot. Met. Phys. Chem. 2017, 53, 495–502. [Google Scholar] [CrossRef]
- Dehnavi, V.; Luan, B.L.; Liu, X.Y.; Shoesmith, D.W.; Rohani, S. Correlation between plasma electrolytic oxidation treatment stages and coating microstructure on aluminum under unipolar pulsed DC mode. Surf. Coat. Technol. 2014, 269, 91–99. [Google Scholar] [CrossRef]
- Shkirskiy, V.; King, A.D.; Gharbi, O.; Volovitch, P.; Scully, J.R.; Ogle, K.; Birbilis, N. Revisiting the electrochemical impedance spectroscopy of magnesium with online inductively coupled plasma atomic emission spectroscopy. Chem. Phys. Chem. 2015, 16, 536–539. [Google Scholar] [CrossRef] [PubMed]














| Specimen Codes | Electrical Parameters | Waveform Shapes |
|---|---|---|
| U | Frequency (kHz) = 1 |  |
| Anodic Duty Cycle = 10% | ||
| Cathodic Duty Cycle = − | ||
| B1 | Frequency (kHz) = 1 |  |
| Anodic Duty Cycle = 10% | ||
| Cathodic Duty Cycle = 10% | ||
| B2 | Frequency (kHz) = 1 |  |
| Anodic Duty Cycle = 10% | ||
| Cathodic Duty Cycle = 20% | ||
| B3 | Frequency (kHz) = 1 |  |
| Anodic Duty Cycle = 10% | ||
| Cathodic Duty Cycle = 30% |
| Specimen | Surface Porosity (%) | Average Pores Size (μm) |
|---|---|---|
| U | 7.7 | 3.2 |
| B1 | 3.1 | 2.8 |
| B2 | 2.6 | 2.8 |
| B3 | 2.9 | 3 |
| Waveform Types | Element (at. %) | ||||||
|---|---|---|---|---|---|---|---|
| Mg | Al | O | F | Si | Na | K | |
| U | 30.7 | 2.0 | 43.5 | 8.1 | 13.5 | 1.9 | 0.3 |
| B1 | 31.1 | 2.9 | 42.4 | 7.6 | 13.5 | 2.1 | 0.4 |
| B2 | 31.9 | 2.9 | 41.5 | 7.3 | 13.7 | 2.3 | 0.4 |
| B3 | 30.9 | 2.8 | 41.7 | 8.1 | 13.7 | 2.4 | 0.4 |
| Specimens | Immersion Time | Outer Layer | Inner layer | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CPE (μF·cm−2·Sn−1) | n | R (kΩ·cm2) | CPE (μF·cm−2·Sn−1) | n | R (kΩ·cm2) | Rl (kΩ·cm2) | L (kH·cm2) | ||
| U | 4-day | 0.24 ± 0.02 | 0.81 ± 0.01 | 250.01 ± 0.53 | 0.36 ± 0.03 | 0.72 ± 0.01 | 1184 ± 0.14 | − | − |
| 1-week | 0.19 ± 0.01 | 0.86 ± 0.02 | 0.56 ± 0.02 | 0.09 ± 0.02 | 0.90 ± 0.07 | 20 ± 0.05 | − | − | |
| 3-week | − | − | − | 3.91 ± 0.03 | 0.89 ± 0.07 | 3 ± 0.01 | 5.211 ± 54.00 | 0.98 ± 0.03 | |
| B1 | 4-day | 0.23 ± 0.03 | 0.84 ± 0.09 | 342.80 ± 0.58 | 1.21 ± 0.26 | 0.81 ± 0.07 | 1010 ± 0.11 | − | − |
| 1-week | 0.74 ± 0.03 | 0.84 ± 0.02 | 1.17 ± 0.07 | 0.30 ± 0.12 | 0.98 ± 0.05 | 12 ± 0.34 | − | − | |
| 3-week | 1.04 ± 0.04 | 0.99 ± 0.03 | 0.09 ± 0.04 | 2.57 ± 0.03 | 0.90 ± 0.07 | 5 ± 0.00 | 15.98 ± 1.44 | 6.41 ± 0.06 | |
| B2 | 4-day | 0.49 ± 0.04 | 0.85 ± 0.03 | 749.00 ± 0.08 | 1.51 ± 0.03 | 0.76 ± 0.07 | 1142 ± 0.13 | − | − |
| 1-week | 0.27 ± 0.03 | 0.89 ± 0.02 | 0.39 ± 0.07 | 0.31 ± 0.12 | 0.85 ± 0.05 | 102 ± 0.12 | − | − | |
| 3-week | 0.60 ± 0.04 | 0.99 ± 0.03 | 0.15 ± 0.48 | 1.72 ± 0.03 | 0.90 ± 0.07 | 7 ± 0.1 | 10.44 ± 1.44 | 5.62 ± 0.06 | |
| B3 | 4-day | 0.35 ± 0.09 | 0.80 ± 0.06 | 394.40 ± 0.08 | 0.59 ± 0.03 | 0.85 ± 0.08 | 1119 ± 0.02 | − | − |
| 1-week | 0.57 ± 0.03 | 0.79 ± 0.02 | 1.12 ± 0.07 | 0.04 ± 0.12 | 0.97 ± 0.05 | 115 ± 0.01 | − | − | |
| 3-week | 1.10 ± 0.04 | 0.99 ± 0.03 | 0.12 ± 0.48 | 1.96 ± 0.03 | 0.91 ± 0.07 | 8 ± 0.01 | 17.39 ± 1.44 | 6.04 ± 0.04 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmati, M.; Raeissi, K.; Toroghinejad, M.R.; Hakimizad, A.; Santamaria, M. Effect of Pulse Current Mode on Microstructure, Composition and Corrosion Performance of the Coatings Produced by Plasma Electrolytic Oxidation on AZ31 Mg Alloy. Coatings 2019, 9, 688. https://doi.org/10.3390/coatings9100688
Rahmati M, Raeissi K, Toroghinejad MR, Hakimizad A, Santamaria M. Effect of Pulse Current Mode on Microstructure, Composition and Corrosion Performance of the Coatings Produced by Plasma Electrolytic Oxidation on AZ31 Mg Alloy. Coatings. 2019; 9(10):688. https://doi.org/10.3390/coatings9100688
Chicago/Turabian StyleRahmati, Maryam, Keyvan Raeissi, Mohammad Reza Toroghinejad, Amin Hakimizad, and Monica Santamaria. 2019. "Effect of Pulse Current Mode on Microstructure, Composition and Corrosion Performance of the Coatings Produced by Plasma Electrolytic Oxidation on AZ31 Mg Alloy" Coatings 9, no. 10: 688. https://doi.org/10.3390/coatings9100688