Novel Perimidine Derivatives as Corrosion Inhibitors of HRB400 Steel in Simulated Concrete Pore Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Inhibitors
2.1.1. 1H-perimidine (PMD)
2.1.2. 1H-Perimidine-2-thiol (SPMD)
2.2. Materials
2.3. Electrochemical Measurements
2.4. Surface Morphological Test and Contact Angle Analysis
2.5. Quantum Chemical Calculations
3. Results and Discussion
3.1. Electrochemical Experiments
3.1.1. Potentiodynamic Polarization (PDP)
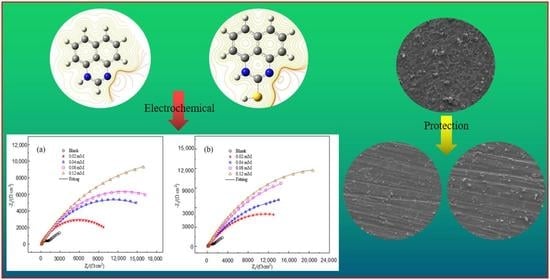
3.1.2. EIS
3.2. Adsorption Isotherm and Thermodynamic Calculation
3.3. Surface Morphology
3.4. Contact Angle and Surface Free Energy
3.5. Quantum Chemical Calculations
3.6. Mechanism of Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhi, F.; Jiang, L.; Jin, M.; Xu, P.; Xiao, B.; Jiang, Q.; Chen, L.; Gu, Y. Inhibition effect and mechanism of polyacrylamide for steel corrosion in simulated concrete pore solution. Constr. Build. Mater. 2020, 259, 120425. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, A.; Zhang, L.; Liu, J.; Han, Y.; Shu, H.; Wang, J. Study on the influence of compound rust inhibitor on corro-sion of steel bars in chloride concrete by electrical parameters. Constr. Build. Mater. 2020, 262, 120763–120777. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.; Ebenso, E.E. Quinoline and its derivatives as corrosion inhibitors: A review. Surf. Interfaces 2020, 21, 100634. [Google Scholar] [CrossRef]
- Thompson, N.G.; Yunovich, M.; Dunmire, D. Cost of corrosion and corrosion maintenance strategies. Corros. Rev. 2007, 25, 247–262. [Google Scholar] [CrossRef]
- Fajardo, G.; Escadeillas, G.; Arliguie, G. Electrochemical chloride extraction (ECE) from steel-reinforced concrete specimens contaminated by “artificial” sea-water. Corros. Sci. 2006, 48, 110–125. [Google Scholar] [CrossRef]
- Bastidas, D.; Criado, M.; La Iglesia, V.; Fajardo, S.; La Iglesia, A. Comparative study of three sodium phosphates as corrosion inhibitors for steel reinforcements. Cem. Concr. Compos. 2013, 43, 31–38. [Google Scholar] [CrossRef]
- Pan, C.; Chen, N.; He, J.; Liu, S.; Chen, K.; Wang, P.; Xu, P. Effects of corrosion inhibitor and functional components on the elec-trochemical and mechanical properties of concrete subject to chloride environment. Constr. Build. Mater. 2020, 260, 119724–119737. [Google Scholar] [CrossRef]
- Sánchez, M.; Alonso, M. Electrochemical chloride removal in reinforced concrete structures: Improvement of effectiveness by simultaneous migration of calcium nitrite. Constr. Build. Mater. 2011, 25, 873–878. [Google Scholar] [CrossRef]
- Valcarce, M.B.; Vazquez, M. Carbon steel passivity examined in alkaline solutions: The effect of chloride and nitrite ions. Electrochim. Acta 2008, 53, 5007–5015. [Google Scholar] [CrossRef]
- Shukla, S.K.; Quraishi, M.; Prakash, R. A self-doped conducting polymer “polyanthranilic acid”: An efficient corrosion inhibitor for mild steel in acidic solution. Corros. Sci. 2008, 50, 2867–2872. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.; Sanaei, Z. Adsorption mechanism and synergistic corrosion-inhibiting effect between the green Nettle leaves extract and Zn2+ cations on carbon steel. J. Ind. Eng. Chem. 2019, 77, 323–343. [Google Scholar] [CrossRef]
- Feng, L.; Yang, H.; Wang, F. Experimental and theoretical studies for corrosion inhibition of carbon steel by imidazoline deriv-ative in 5% NaCl saturated Ca(OH)2 solution. Electrochim. Acta 2011, 58, 427–436. [Google Scholar] [CrossRef]
- He, X.; Mao, J.; Ma, Q.; Tang, Y. Corrosion inhibition of perimidine derivatives for mild steel in acidic media: Electrochemical and computational studies. J. Mol. Liq. 2018, 269, 260–268. [Google Scholar] [CrossRef]
- Herbert, J.; Woodgate, P.; Denny, W. Potential antitumor agents. 53. Synthesis, DNA binding properties, and biological activity of perimidines designed as minimal DNA-intercalating agents. J. Med. Chem. 1987, 30, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Sturla, S.J. A Synthetic Nucleoside Probe that Discerns a DNA Adduct from Unmodified DNA. J. Am. Chem. Soc. 2007, 129, 4882–4883. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, J.; Li, G.; Wang, P.; Wang, P.; Li, F.; Zhang, B.; Chi, H. Corrosion inhibition efficiency of compound nitrite with D-sodium gluconate on carbon steel in simulated concrete pore solution. Constr. Build. Mater. 2021, 288, 123101. [Google Scholar] [CrossRef]
- Sundaram, R.G.; Vengatesh, G.; Sundaravadivelu, M. Surface morphological and quantum chemical studies of some expired drug molecules as potential corrosion inhibitors for mild steel in chloride medium. Surf. Interfaces 2020, 22, 100841. [Google Scholar] [CrossRef]
- Saha, S.; Banerjee, P. A theoretical approach to understand the inhibition mechanism of steel corrosion with two aminoben-zonitrile inhibitors. RSC Adv. 2015, 5, 71120–71130. [Google Scholar] [CrossRef]
- Guo, L.; Safi, Z.; Shi, W.; Tüzün, B.; Altunay, N.; Kaya, C. Anticorrosive effects of some thiophene derivatives against the corrosion of iron: A computational study. Front. Chem. 2018, 6, 155–167. [Google Scholar] [CrossRef]
- Dohare, P.; Ansari, K.; Quraishi, M.; Obot, I. Pyranpyrazole derivatives as novel corrosion inhibitors for mild steel useful for industrial pickling process: Experimental and Quantum Chemical study. J. Ind. Eng. Chem. 2017, 52, 197–210. [Google Scholar] [CrossRef]
- Haque, J.; Ansari, K.; Srivastava, V.; Quraishi, M.; Obot, I. Pyrimidine derivatives as novel acidizing corrosion inhibitors for N80 steel useful for petroleum industry: A combined experimental and theoretical approach. J. Ind. Eng. Chem. 2017, 49, 176–188. [Google Scholar] [CrossRef]
- Jiang, S.; Jiang, L.; Wang, Z.; Jin, M.; Bai, S.; Song, S.; Yan, X. Deoxyribonucleic acid as an inhibitor for chloride-induced corro-sion of reinforcing steel in simulated concrete pore solutions. Constr. Build. Mater. 2017, 150, 238–247. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, Y.; Tang, Y. Inhibition effect and mechanism of sodium oleate on passivation and pitting corrosion of steel in simulated concrete pore solution. Constr. Build. Mater. 2018, 167, 197–204. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, H.; Wang, F. Investigation on the inhibition behavior of a pentaerythritol glycoside for carbon steel in 3.5% NaCl saturated Ca(OH)2 solution. Corros. Sci. 2012, 54, 193–200. [Google Scholar] [CrossRef]
- Sanaei, Z.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1 M HCl solution: A complementary experimental, molecular dynamics and quantum mechanics investigation. J. Ind. Eng. Chem. 2019, 69, 18–31. [Google Scholar] [CrossRef]
- Azeez, F.A.; Al-Rashed, O.A.; Nazeer, A.A. Controlling of mild-steel corrosion in acidic solution using environmentally friendly ionic liquid inhibitors: Effect of alkyl chain. J. Mol. Liq. 2018, 265, 654–663. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, T.; Yu, X.; Chen, D. Corrosion inhibition efficiency of triethanolammonium dodecylbenzene sulfonate on Q235 carbon steel in simulated concrete pore solution. Corros. Sci. 2019, 158, 108097–108109. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Nishihara, H.; Aramaki, K. The inhibition of passive film breakdown on iron in a borate buffer solution con-taining chloride ions by organic anion inhibitors. Corros. Sci. 1994, 36, 241–258. [Google Scholar] [CrossRef]
- Valek, L.; Martinez, S.; Mikulić, D.; Brnardić, I. The inhibition activity of ascorbic acid towards corrosion of steel in alkaline media containing chloride ions. Corros. Sci. 2008, 50, 2705–2709. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Nishihara, H.; Aramaki, K. The inhibition of pit growth on an iron surface in a borate buffer solution containing chloride ion by inhibitors classified as soft bases in the HSAB principle. Corros. Sci. 1995, 37, 571–585. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, M.; Zheng, J.; Castaneda, H. Corrosion inhibition of mild steel by an imidazolium ionic liquid compound: The effect of pH and surface pre-corrosion. RSC Adv. 2015, 5, 95160–95170. [Google Scholar] [CrossRef]
- Hu, J.; Koleva, D.; Petrov, P.; Breugel, K. Polymeric vesicles for corrosion control in reinforced mortar: Electrochemical behav-ior, steel surface analysis and bulk matrix properties. Corros. Sci. 2012, 65, 414–430. [Google Scholar] [CrossRef]
- John, D.G.; Searson, P.C.; Dawson, J.L. Use of AC impedance technique in studies on steel in concrete in immersed conditions. Br. Corros. J. 1981, 16, 102–106. [Google Scholar] [CrossRef]
- Ansari, K.; Quraishi, M. Bis-Schiff bases of isatin as new and environmentally benign corrosion inhibitor for mild steel. J. Ind. Eng. Chem. 2014, 20, 2819–2829. [Google Scholar] [CrossRef]
- Al-Mehthel, M.; Al-Dulaijan, S.; Al-Idi, S.H.; Shameem, M.; Ali, M.; Maslehuddin, M. Performance of generic and proprietary corrosion inhibitors in chloride-contaminated silica fume cement concrete. Constr. Build. Mater. 2009, 23, 1768–1774. [Google Scholar] [CrossRef]
- Gunay, H.B.; Ghods, P.; Isgor, O.B.; Carpenter, G.J.; Wu, X. Characterization of atomic structure of oxide films on carbon steel in simulated concrete pore solutions using EELS. Appl. Surf. Sci. 2013, 274, 195–202. [Google Scholar] [CrossRef]
- Lebrini, M.; Suedile, F.; Salvin, P.; Roos, C.; Zarrouk, A.; Jama, C.; Bentiss, F. Bagassa guianensis ethanol extract used as sustain-able eco-friendly inhibitor for zinc corrosion in 3% NaCl: Electrochemical and XPS studie. Surf. Interfaces 2020, 20, 100588–100603. [Google Scholar] [CrossRef]
- Hemapriya, V.; Prabakaran, M.; Parameswari, K.; Chitra, S.; Kim, S.; Chung, I. Dry and wet lab analysis on benzofused hetero-cyclic compounds as effective corrosion inhibitors for mild steel in acidic medium. J. Ind. Eng. Chem. 2016, 40, 106–117. [Google Scholar] [CrossRef]
- Ma, Q.; Qi, S.; He, X.; Tang, Y.; Lu, G. 1,2,3-Triazole derivatives as corrosion inhibitors for mild steel in acidic medium: Experimental and computational chemistry studies. Corros. Sci. 2017, 129, 91–101. [Google Scholar] [CrossRef]
- Herrag, L.; Hammouti, B.; Elkadiri, S.; Aouniti, A.; Jama, C.; Vezin, H.; Bentiss, F. Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: Experimental and theoretical investigations. Corros. Sci. 2010, 52, 3042–3051. [Google Scholar] [CrossRef]
- Zhang, Z.; Ba, H.; Wu, Z. Sustainable corrosion inhibitor for steel in simulated concrete pore solution by maize gluten meal extract: Electrochemical and adsorption behavior studies. Constr. Build. Mater. 2019, 227, 117080. [Google Scholar] [CrossRef]
- Alinejad, S.; Naderi, R.; Mahdavian, M. Effect of inhibition synergism of zinc chloride and 2-mercaptobenzoxzole on protec-tive performance of an ecofriendly silane coating on mild steel. J. Ind. Eng. Chem. 2017, 48, 88–98. [Google Scholar] [CrossRef]
- Li, D.; Neumann, A.W. A reformulation of the equation of state for interfacial tensions. J. Colloid Interface Sci. 1990, 137, 304–307. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, B.; Liu, Y.; Yang, W.; Yin, X.; Chen, Y.; Le, J.; Chen, Z. Corrosion inhibition properties of two imidazolium ionic liq-uids with hydrophilic tetrafluoroborate and hydrophobic hexafluorophosphate anions in acid medium. J. Ind. Eng. Chem. 2017, 56, 234–247. [Google Scholar] [CrossRef]
- Pandarinathan, V.; Lepková, K.; Bailey, S.I.; Becker, T.; Gubner, R. Adsorption of corrosion inhibitor 1-Dodecylpyridinium chloride on carbon steel studied by in Situ AFM and electrochemical methods. Ind. Eng. Chem. Res. 2014, 53, 5858–5865. [Google Scholar] [CrossRef]
- Hejazi, S.; Mohajernia, S.; Moayed, M.; Davoodi, A.; Rahimizadeh, M.; Momeni, M.; Eslami, A.; Shiri, A.; Kosari, A. Electrochem-ical and quantum chemical study of Thiazolo-pyrimidine derivatives as corrosion inhibitors on mild steel in 1 M H2SO4. J. Ind. Eng. Chem. 2015, 25, 112–121. [Google Scholar] [CrossRef]
- Alrefaee, S.H.; Rhee, K.Y.; Verma, C.; Quraishi, M.; Ebenso, E.E. Challenges and advantages of using plant extract as inhibitors in modern corrosion inhibition systems: Recent advancements. J. Mol. Liq. 2020, 321, 114666. [Google Scholar] [CrossRef]
- Obi-Egbedi, N.; Obot, I. Inhibitive properties, thermodynamic and quantum chemical studies of alloxazine on mild steel corro-sion in H2SO4. Corros. Sci. 2011, 53, 263–275. [Google Scholar] [CrossRef]
- Eddy, N.; Awe, F.; Gimba, C.; Ibisi, N.; Ebenso, E. Experimental and Computational Chemistry Simulation Studies on the inhi-bition potentials of some amino acids for the corrosion of mild steel in 0.1 M. Int. J. Electrochem. Sci. 2011, 6, 931–957. [Google Scholar]
- Machnikova, E.; Whitmire, K.H.; Hackerman, N. Corrosion inhibition of carbon steel in hydrochloric acid by furan derivatives. Electrochim. Acta 2008, 53, 6024–6032. [Google Scholar] [CrossRef]
- Masoud, M.; Awad, M.; Shaker, M.; El-Tahawy, M. The role of structural chemistry in the inhibitive performance of some aminopyrimidines on the corrosion of steel. Corros. Sci. 2010, 52, 2387–2396. [Google Scholar] [CrossRef]
- Berdimurodov, E.; Kholikovb, A.; Akbarov, K.; Guo, L. Inhibition properties of 4,5-dihydroxy-4,5-di-p-tolylimidazolidine-2-thione for use on carbon steel in an aggressive alkaline medium with chloride ions: Thermodynamic, electrochemical, surface and theoretical analyses. J. Mol. Liq. 2021, 327, 114813–114830. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.; Quraishi, M.; Rhee, K. Phthalocyanine, naphthalocyanine and their derivatives as Corrosion Inhibi-tors: A review. J. Mol. Liq. 2021, 334, 116441–116449. [Google Scholar] [CrossRef]









| Inhibitors | Conc. | Ecorr | Epit | −βc | βa | Icorr | IE | θ |
|---|---|---|---|---|---|---|---|---|
| mM | mv | mv | mV dec−1 | mV dec−1 | μA cm−2 | % | ||
| Blank | −658.81 ± 5.50 | −73.66 ± 3.15 | 197.24 ± 3.96 | 251.39 ± 4.56 | 23.69 ± 1.15 | / | / | |
| PMD | 0.02 | −486.62 ± 4.75 | −26.89 ± 2.78 | 177.24 ± 4.51 | 196.00 ± 4.12 | 12.97 ± 1.01 | 45.25 | 0.4525 |
| 0.04 | −415.19 ± 6.23 | 55.69 ± 1.96 | 168.08 ± 3.62 | 194.10 ± 4.37 | 9.28 ± 1.37 | 60.84 | 0.6084 | |
| 0.08 | −350.40 ± 5.66 | 132.46 ± 3.05 | 170.54 ± 3.74 | 187.13 ± 3.95 | 6.24 ± 0.86 | 73.66 | 0.7366 | |
| 0.12 | −330.47 ± 4.97 | 194.15 ± 2.94 | 164.85 ± 3.55 | 189.15 ± 4.16 | 3.68 ± 0.65 | 84.48 | 0.8448 | |
| SPMD | 0.02 | −435.85 ± 7.32 | 53.94 ± 1.75 | 167.17 ± 4.09 | 187.31 ± 5.03 | 10.43 ± 1.27 | 55.99 | 0.5599 |
| 0.04 | −408.14 ± 6.40 | 91.32 ± 2.46 | 170.88 ± 4.27 | 190.54 ± 4.48 | 7.36 ± 0.79 | 68.95 | 0.6895 | |
| 0.08 | −343.98 ± 5.73 | 130.06 ± 3.54 | 166.43 ± 3.05 | 193.39 ± 3.72 | 5.07 ± 0.93 | 78.60 | 0.7860 | |
| 0.12 | −315.49 ± 5.49 | 175.99 ± 3.17 | 163.39 ± 3.96 | 186.27 ± 3.85 | 2.94 ± 0.82 | 87.61 | 0.8761 |
| Cinh/(mM) | Rs | CPE1 | Rf | CPE2 | Rct | IE | |||
|---|---|---|---|---|---|---|---|---|---|
| Ω cm2 | Y0 (μΩ−1S−ncm−2) | n1 | kΩ cm2 | Y0 (μΩ−1S−ncm−2) | n2 | kΩ cm2 | % | ||
| Blank | 9.89 ± 1.01 | 410.9 ± 3.5 | 0.89 ± 0.03 | 0.41 ± 0.04 | 1 378.1 ± 10.5 | 0.61 ± 0.05 | 6.94 ± 0.52 | / | |
| PED | 0.02 | 30.05 ± 2.25 | 306.5 ± 3.0 | 0.93 ± 0.05 | 0.59 ± 0.03 | 783.5 ± 10.8 | 0.65 ± 0.03 | 11.95 ± 0.69 | 41.92 |
| 0.04 | 64.03 ± 2.53 | 247.1 ± 2.2 | 0.84 ± 0.07 | 1.32 ± 0.05 | 512.2 ± 6.2 | 0.59 ± 0.04 | 17.01 ± 0.83 | 59.20 | |
| 0.08 | 75.18 ± 2.45 | 179.8 ± 2.3 | 0.88 ± 0.08 | 2.01 ± 0.02 | 405.6 ± 8.5 | 0.56 ± 0.05 | 25.28 ± 0.75 | 72.55 | |
| 0.12 | 82.95 ± 2.54 | 105.2 ± 2.0 | 0.90 ± 0.04 | 5.64 ± 0.03 | 150.8 ± 7.2 | 0.62 ± 0.03 | 35.77 ± 0.66 | 80.60 | |
| SPED | 0.02 | 37.81 ± 1.97 | 279.6 ± 1.8 | 0.82 ± 0.07 | 1.05 ± 0.06 | 660.9 ± 5.6 | 0.67 ± 0.02 | 15.12 ± 0.72 | 54.10 |
| 0.04 | 70.23 ± 2.66 | 198.5 ± 1.9 | 0.85 ± 0.03 | 3.09 ± 0.02 | 435.7 ± 4.8 | 0.66 ± 0.04 | 22.94 ± 0.35 | 69.75 | |
| 0.08 | 81.23 ± 2.74 | 109.1 ± 2.0 | 0.87 ± 0.06 | 6.07 ± 0.05 | 278.5 ± 3.9 | 0.59 ± 0.03 | 29.14 ± 0.46 | 76.18 | |
| 0.12 | 96.16 ± 2.83 | 65.7 ± 1.3 | 0.88 ± 0.05 | 9.01 ± 0.06 | 99.4 ± 4.3 | 0.65 ± 0.06 | 48.85 ± 0.58 | 85.79 | |
| Inhibitors | Kads (L·mol −1) | (kJ/mol) |
|---|---|---|
| PMD | 38,240.9 | −36.11 |
| SPMD | 58,858.2 | −37.18 |
| Blank | PMD | SPMD | |
|---|---|---|---|
| α (deg) | 27 | 61 | 66 |
| γsv (mJ/m2) | 65.15 | 46.62 | 43.59 |
| Compounds | EHOMO/ev | ELUMO/ev | ΔE/ev | μ/(Debye) | χ | η | ΔN |
|---|---|---|---|---|---|---|---|
| PMD | −4.88 | −0.64 | 4.24 | 3.02 | 2.758 | 2.12 | 1.00 |
| SPMD | −4.96 | −0.85 | 4.11 | 3.37 | 2.906 | 2.05 | 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Cai, J.; Mu, S.; Zhang, H.; Liu, K.; Liu, J.; Hong, J. Novel Perimidine Derivatives as Corrosion Inhibitors of HRB400 Steel in Simulated Concrete Pore Solution. Coatings 2023, 13, 73. https://doi.org/10.3390/coatings13010073
Ma Q, Cai J, Mu S, Zhang H, Liu K, Liu J, Hong J. Novel Perimidine Derivatives as Corrosion Inhibitors of HRB400 Steel in Simulated Concrete Pore Solution. Coatings. 2023; 13(1):73. https://doi.org/10.3390/coatings13010073
Chicago/Turabian StyleMa, Qi, Jingshun Cai, Song Mu, Hao Zhang, Kai Liu, Jianzhong Liu, and Jinxiang Hong. 2023. "Novel Perimidine Derivatives as Corrosion Inhibitors of HRB400 Steel in Simulated Concrete Pore Solution" Coatings 13, no. 1: 73. https://doi.org/10.3390/coatings13010073