Influence of Post-Deposition Thermal Treatments on the Morpho-Structural, and Bonding Strength Characteristics of Lithium-Doped Biological-Derived Hydroxyapatite Coatings
Abstract
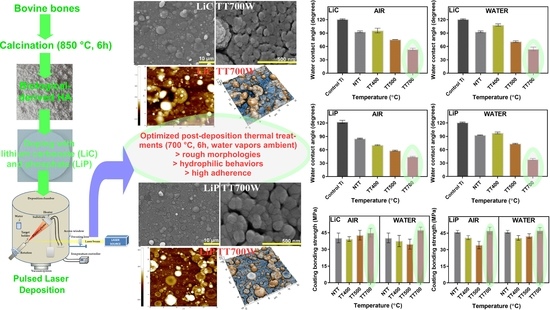
:1. Introduction
2. Materials and Methods
2.1. Powder Preparation
2.2. Target Preparation
2.3. Substrates Preparation
2.4. PLD Coatings
2.5. Post-Deposition Thermal Treatments
2.6. Physical-Chemical and Mechanical Characterization Methods
- (a).
- The crystalline status and phase composition of LiC and LiP coatings were investigated by X-ray diffraction (XRD), in grazing incidence (GI) geometry, using a Rigaku SmartLab (3 kW) equipment, with parallel beam setting and CuKα radiation (λ = 1.5406 Å). The angle of incidence (α) was 2°. The patterns were recorded in the 2θ = (10–60)° range, at an acquisition time of 1°/min.
- (b).
- The chemical structure of LiC and LiP coatings was investigated by Fourier transform infrared (FT-IR) spectroscopy, using a Perkin Elmer BX Spectrum equipment, operated in an attenuated total reflectance (ATR) mode. A Pike-MIRacle diamond head (0.18 cm diameter) was used. All measurements were performed on Ti substrates, in the range (1600–500) cm−1, with a resolution of 4 cm−1 and a total number of 64 scans/experiment.
- (c).
- The surface and bottom-to-top morphologies of LiC and LiP coatings were investigated using SEM, in top- and cross-view modes, respectively. An FEI Inspect S50 electron microscope (Field Electron and Ion Company, Hillsboro, OR, USA), used in the secondary electron mode, at an acceleration voltage of 20 kV, was used. Before examination, to prevent electrical charging, all samples were coated with a thin layer (10–12) nm of Au.
- (d).
- To better emphasize the topological surface peculiarities of LiC and LiP coatings, high resolution Atomic Force Microscopy (AFM), performed in non-contact mode on a XE-100 apparatus from Park Systems, was used. Sharp tips (PPP-NCHR type from NanosensorsTM, Neuchâtel, Switzerland), having a length of 125 µm, a 30 µm width and a radius of curvature of less than 8 nm, were used for surface scanning over areas of (20 × 20) µm2. All AFM measurements were performed at room temperature (RT). The textural (amplitude) parameters, namely average roughness (Rq), surface skewness (Rsk) and surface kurtosis (Rku), were determined.
- (e).
- The chemical composition of LiC and LiP samples was assessed by energy dispersive X-ray spectroscopy (EDS). For these measurements, a SiLi EDAX Inc. detector (Mahwah, NJ, USA), operated at 20 kV, was used. The elemental analyses were performed in triplicate, on different areas ((250 × 250) µm2) of the deposited layers.
- (f).
- The wetting properties of the LiC and LiP coatings were investigated by static (equilibrium) contact angle (CA) measurements, using a Drop Shape Analysis System, model DSA100 (Krüss GmbH, Hamburg, Germany). The samples were positioned on a flat platform, under the tip of a blunt-end, stainless steel needle, with an outer diameter of 0.5 mm. The needle was attached to a syringe pump controlled by the DSA3® PC software and used for dripping liquids with a controlled volume on the test surface and for the CA evaluation. The volume of one liquid droplet was of ~1 μL. For each sample, the measurements were performed on two different regions of the surface. The CA measurements were performed by fitting the experimental profile of the sessile drop with a second-degree polynomial or with the equation of the circle, after which the slope of the tangent to the drop at the point of intersection with the line separating the liquid–solid–vapor interface was calculated. The video camera was tilted at an angle of ~2° to the sample plane. All measurements were performed at RT, in duplicate (on two identical samples). Based on the measured CA values, the solid Surface Free Energy (SFE) was determined. For the CA and SFE measurements, two standard liquids (water and diiodomethane) were used, for which one knows: the surface tension (γwater = 72.8 mN/m; γdiiodmethane = 50.8 mN/m), and the dispersion and polar components (γwaterd = 21.8 mN/m, γwaterp = 51 mN/m, and γdiiodmethaned = 48.5 mN/m, γdiiodmethanep = 2.3 mN/m). Using the CA values obtained for the two testing liquids, both the polar and dispersion parts of the synthesized coatings, and the total SFE, was estimated by applying the Owens–Wendt method [36].
- (g).
- The adherence of the LiC and LiP coatings to the Ti substrate was estimated by the pull-out method. A standardized instrument, PAThandy AT101 (maximum pulling force = 1 kN) from DFD Instruments® (Kristiansand, Norway), equipped with stainless steel test elements with a diameter of Φ = 0.28 cm, was used. The complete procedure was detailed elsewhere [37], and it complies with the ASTM D4541 and ISO 4624 standards. The measurements were performed on quadruplicates.
3. Results and Discussion
3.1. X-ray Diffraction
3.2. Fourier Transformed Infra-Red Spectroscopy
3.3. Scanning Electron Microscopy
3.4. Atomic Force Microscopy
3.5. Energy Dispersive X-ray Spectroscopy
3.6. Contact Angle Measurements
3.7. Pull-Out Bonding Strength Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denry, I.; Kuhn, L.T. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent. Mater. 2016, 32, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Allied Market Research. Dental Implants and Prosthetics Market by Products (Dental Implants, Dental Prosthetics), by Materials (Metals, Polymers, Ceramics, Biomaterial): Global Opportunity Analysis and Industry Forecast, 2020–2030. Available online: https://www.alliedmarketresearch.com/dental-implants-and-prosthetics-market (accessed on 3 November 2022).
- Allied Market Research. Orthopedic Implants Market Expected to Reach $68.80 Billion by 2030. Available online: https://www.alliedmarketresearch.com/press-release/orthopedic-implants-market.html (accessed on 3 November 2022).
- Medany, S.S.; Elkamel, R.S.; Abdel-Gawad, S.A.; Fekry, A.M. A Novel Nano-Composite CSNPs/PVP/CoONPs Coating for Improving Corrosion Resistance of Ti-6Al-4V Alloy as a Dental Implant. Metals 2022, 12, 1784. [Google Scholar] [CrossRef]
- Hulka, I.; Florido-Suarez, N.R.; Mirza-Rosca, J.C.; Saceleanu, A. Mechanical Properties and Corrosion Behavior of Thermally Treated Ti-6Al-7Nb Dental Alloy. Materials 2022, 15, 3813. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Williams, J.C. Perspectives on titanium science and technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Agius, D.; Kourousis, K.I.; Wallbrink, C. A review of the as-built SLM Ti-6Al-4V mechanical properties towards achieving fatigue resistant designs. Metals 2018, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Rau, J.V.; Fadeeva, I.V.; Forysenkova, A.A.; Davydova, G.A.; Fosca, M.; Filippov, Y.Y.; Antoniac, I.V.; Antoniac, A.; D’Arco, A.; Di, M.; et al. Strontium Substituted Tricalcium Phosphate Bone Cement: Short and Long-Term Time-Resolved Studies and In Vitro Properties. Adv. Mater. Interfaces 2022, 9, 2200803. [Google Scholar] [CrossRef]
- Sargeant, A.; Goswami, T. Hip implants-Paper VI-Ion concentrations. Mater. Des. 2007, 28, 155–171. [Google Scholar] [CrossRef]
- Koch, C.; Johnson, S.; Kumar, D.; Jelinek, M.; Chrisey, D.; Doraiswamy, A.; Jin, C.; Narayan, R.J.; Mihailescu, I.N. Pulsed laser deposition of hydroxyapatite thin films. Mater. Sci. Eng. C 2007, 27, 484–494. [Google Scholar] [CrossRef]
- Ananth, H.; Kundapur, V.; Mohammed, H.S.; Anand, M.; Amarnath, G.S.; Mankar, S. A review on biomaterials in dental implantology. Int. J. Biomed. Sci. 2015, 11, 113–120. [Google Scholar]
- Li, J.; Yang, L.; Guo, X.; Cui, W.; Yang, S.; Wang, J.; Qu, Y.; Shao, Z.; Xu, S. Osteogenesis effects of strontium-substituted hydroxyapatite coatings on true bone ceramic surfaces in vitro and in vivo. Biomed. Mater. 2018, 13, 015018. [Google Scholar] [CrossRef]
- Oktar, F.N.; Unal, S.; Gunduz, O.; Nissan, B.B.; Macha, I.J.; Akyol, S.; Duta, L.; Ekren, N.; Altan, E.; Yetmez, M. Marine-derived bioceramics for orthopedic, reconstructive and dental surgery applications. J. Aust. Ceram. Soc. 2022. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Wang, B.; Feng, C.; Liu, Y.; Mi, F.; Dong, J. Recent advances in biofunctional guided bone regeneration materials for repairing defective alveolar and maxillofacial bone: A review. Jpn. Dent. Sci. Rev. 2022, 58, 233–248. [Google Scholar] [CrossRef]
- Qiao, W.; Liu, Q.; Li, Z.; Zhang, H.; Chen, Z. Changes in physicochemical and biological properties of porcine bone derived hydroxyapatite induced by the incorporation of fluoride. Sci. Technol. Adv. Mater. 2017, 18, 110–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Rodriguez, A.P.; Nagai, N.; Nagatsuka, H.; LeGeros, R.Z.; Tsujigiwa, H.; Inoue, M.; Kishimoto, E.; Takagi, S. Effect of Fluoride-substituted Apatite on In Vivo Bone Formation. J. Biomater. Appl. 2011, 25, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Eason, R. Pulsed Laser Deposition of Thin Films: Applications-Led Growth of Functional Materials, 1st ed.; Wiley & Sons Interscience: Hoboken, NJ, USA, 2007; pp. 1–705. [Google Scholar]
- Montazerian, M.; Hosseinzadeh, F.; Migneco, C.; Fook, M.V.L.; Baino, F. Bioceramic coatings on metallic implants: An overview. Ceram. Int. 2022, 48, 8987–9005. [Google Scholar] [CrossRef]
- Duta, L.; Popescu, A.C. Current Status on Pulsed Laser Deposition of Coatings from Animal-Origin Calcium Phosphate Sources. Coatings 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Duta, L. In Vivo Assessment of Synthetic and Biological-Derived Calcium Phosphate-Based Coatings Fabricated by Pulsed Laser Deposition: A Review. Coatings 2021, 11, 99. [Google Scholar] [CrossRef]
- Duta, L.; Oktar, F.N.; Stan, G.E.; Popescu-Pelin, G.; Serban, N.; Luculescu, C.; Mihailescu, I.N. Novel doped hydroxyapatite thin films obtained by pulsed laser deposition. Appl. Surf. Sci. 2013, 265, 41–49. [Google Scholar] [CrossRef]
- Graziani, G.; Boi, M.; Bianchi, M. A Review on Ionic Substitutions in Hydroxyapatite Thin Films: Towards Complete Biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- Farmani, A.R.; Salmeh, M.A.; Golkar, Z.; Moeinzadeh, A.; Ghiasi, F.F.; Amirabad, S.Z.; Shoormeij, M.H.; Mahdavinezhad, F.; Momeni, S.; Moradbeygi, F.; et al. Li-Doped Bioactive Ceramics: Promising Biomaterials for Tissue Engineering and Regenerative Medicine. J. Funct. Biomater. 2022, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Duta, L.; Chifiriuc, M.C.; Popescu-Pelin, G.; Bleotu, C.; Gradisteanu, G.(P.); Anastasescu, M.; Achim, A.; Popescu, A. Pulsed Laser Deposited Biocompatible Lithium-Doped Hydroxyapatite Coatings with Antimicrobial Activity. Coatings 2019, 9, 54. [Google Scholar] [CrossRef] [Green Version]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of Metals for Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, I.; Berger, U.; Markitziu, A.; Gidalia, I. The uptake of lithium ions by synthetic carbonated hydroxyapatite. Calcif. Tissue Int. 1986, 38, 293–295. [Google Scholar] [CrossRef]
- Koutsoukos, P.G.; Nancollas, G.H. The effect of lithium on the precipitation of hydroxyapatite from aqueous solutions. Colloids Surf. 1986, 17, 361–370. [Google Scholar] [CrossRef]
- Shainberg, A.P.M.; Valério, P.; Zonari, A.; Oktar, F.N.; Ozyegin, L.S.; Graça, M.P.F.; Leite, M.F.; Goes, A.M. Attachment and proliferation of osteoblasts on lithium-hydroxyapatite composites. Adv. Mater. Sci. Eng. 2012, 2012, 650574. [Google Scholar] [CrossRef] [Green Version]
- Popescu, A.C.; Florian, P.E.; Stan, G.E.; Popescu-Pelin, G.; Zgura, I.; Enculescu, M.; Oktar, F.N.; Trusca, R.; Sima, L.E.; Roseanu, A.; et al. Physical-chemical characterization and biological assessment of simple and lithium-doped biological-derived hydroxyapatite thin films for a new generation of metallic implants. Appl. Surf. Sci. 2018, 439, 724–735. [Google Scholar] [CrossRef]
- Florian, P.E.; Duta, L.; Grumezescu, V.; Popescu-Pelin, G.; Popescu, A.C.; Oktar, F.N.; Evans, R.W.; Constantinescu, A.R. Lithium-Doped Biological-Derived Hydroxyapatite Coatings Sustain In Vitro Differentiation of Human Primary Mesenchymal Stem Cells to Osteoblasts. Coatings 2019, 9, 781. [Google Scholar] [CrossRef] [Green Version]
- Sakudo, A. Inactivation Methods for Prions. Curr. Issues Mol. Biol. 2020, 36, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Chioibasu, D.; Achim, A.; Popescu, C.; Stan, G.E.; Pasuk, I.; Enculescu, M.; Iosub, S.; Duta, L.; Popescu, A. Prototype Orthopedic Bone Plates 3D Printed by Laser Melting Deposition. Materials 2019, 12, 906. [Google Scholar] [CrossRef] [PubMed]
- Stan, G.E.; Marcov, D.A.; Popa, A.C.; Husanu, M.A. Polymer-like and diamond-like carbon coatings prepared by RF-PECVD for biomedical applications. Dig. J. Nanomater. Biostruct. 2010, 5, 705–718. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Popa, A.C.; Stan, G.E.; Husanu, M.A.; Pasuk, I.; Popescu, I.D.; Popescu, A.C.; Mihailescu, I.N. Multi-layer haemocompatible diamond-like carbon coatings obtained by combined radio frequency plasma enhanced chemical vapor deposition and magnetron sputtering. J. Mater. Sci. Mater. Med. 2013, 24, 2695–2707. [Google Scholar] [CrossRef] [PubMed]
- Stan, G.E.; Morosanu, C.O.; Marcov, D.A.; Pasuk, I.; Miculescu, F.; Reumont, G. Effect of annealing upon the structure and adhesion properties of sputtered bio-glass/titanium coatings. Appl. Surf. Sci. 2009, 255, 9132–9138. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef] [Green Version]
- Pasteris, J.D.; Wopenka, B.; Freeman, J.J.; Rogers, K.; Valsami-Jones, E.; van der Houwen, J.A.M.; Silva, M.J. Lack of OH in nanocrystalline apatite as a function of degree of atomic order: Implications for bone and biomaterials. Biomaterials 2004, 25, 229–238. [Google Scholar] [CrossRef]
- Cihlář, J.; Buchal, A.; Trunec, M. Kinetics of thermal decomposition of hydroxyapatite bioceramics. J. Mater. Sci. 1999, 34, 6121–6131. [Google Scholar] [CrossRef]
- Komur, B.; Lohse, T.; Can, H.M.; Khalilova, G.; Geçimli, Z.N.; Aydoğdu, M.O.; Kalkandelen, C.; Stan, G.E.; Sahin, Y.M.; Sengil, A.Z.; et al. Fabrication of naturel pumice/hydroxyapatite composite for biomedical engineering. Bio. Med. Eng. OnLine 2016, 15, 81. [Google Scholar] [CrossRef] [Green Version]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
- Markovic, M.; Fowler, B.O.; Tung, M.S. Preparation and Comprehensive Characterization of a Calcium Hydroxyapatite Reference Material. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Chirică, I.M.; Enciu, A.-M.; Tite, T.; Dudău, M.; Albulescu, L.; Iconaru, S.L.; Predoi, D.; Pasuk, I.; Enculescu, M.; Radu, C.; et al. The Physico-Chemical Properties and Exploratory Real-Time Cell Analysis of Hydroxyapatite Nanopowders Substituted with Ce, Mg, Sr, and Zn (0.5–5 at.%). Materials 2021, 14, 3808. [Google Scholar] [CrossRef] [PubMed]
- Gadaleta, S.J.; Paschalis, E.P.; Betts, F.; Mendelsohn, R.; Boskey, A.L. Fourier transform infrared spectroscopy of the solution-mediated conversion of amorphous calcium phosphate to hydroxyapatite: New correlations between X-ray diffraction and infrared data. Calcif. Tissue Int. 1996, 58, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Querido, W.; Shanas, N.; Bookbinder, S.; Oliveira-Nunes, M.C.; Krynska, B.; Pleshko, N. Fourier transform infrared spectroscopy of developing bone mineral: From amorphous precursor to mature crystal. Analyst 2020, 145, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Sima, L.E.; Stan, G.E.; Morosanu, C.O.; Melinescu, A.; Ianculescu, A.; Melinte, R.; Neamtu, J.; Petrescu, S.M. Differentiation of mesenchymal stem cells onto highly adherent radio frequency-sputtered carbonated hydroxylapatite thin films. J. Biomed. Mater. Res. A 2010, 95A, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.; Grossin, D.; Stefan, N.; Duta, L.; Miroiu, F.M.; Stan, G.E.; Sopronyi, M.; Luculescu, C.; Freche, M.; Marsan, O.; et al. Biomimetic nanocrystalline apatite coatings synthesized by matrix assisted pulsed laser evaporation for medical applications. Mater. Sci. Eng. B 2014, 181, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Spence, G.; Phillips, S.; Campion, C.; Brooks, R.; Rushton, N. Bone formation in a carbonate-substituted hydroxyapatite implant is inhibited by zoledronate. J. Bone Joint Surg. Br. 2008, 90-B, 1635–1640. [Google Scholar] [CrossRef]
- Germaini, M.M.; Detsch, R.; Grünewald, A.; Magnaudeix, A.; Lalloue, F.; Boccaccini, A.R.; Champion, E. Osteoblast and osteoclast responses to A/B type carbonate-substituted hydroxyapatite ceramics for bone regeneration. Biomed. Mater. 2017, 12, 035008. [Google Scholar] [CrossRef]
- Peng, G.W.; Chen, S.K.; Liu, H.S. Infrared Absorption Spectra and Their Correlation with the Ti-O Bond Length Variations for TiO2 (Rutile), Na-Titanates, and Na-Titanosilicate (Natisite, Na2TiOSiO4). App. Spectrosc. 1995, 49, 1646–1651. [Google Scholar] [CrossRef]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Lescoute, E.; Hallo, L.; Hébert, D.; Chimier, B.; Etchessahar, B.; Tikhonchuk, V.T.; Chevalier, J.M.; Combis, P. Experimental observations and modeling of nanoparticle formation in laser-produced expanding plasma. Phys. Plasmas 2008, 15, 063507. [Google Scholar] [CrossRef] [Green Version]
- Mihailescu, I.N.; Ristoscu, C.; Bigi, A.; Mayer, I. Advanced biomimetic implants based on nanostructured coatings synthesized by pulsed laser technologies. In Laser-Surface Interactions for New Materials Production, Tailoring Structure and Properties; Miotello, A., Ossi, M., Eds.; Springer: New York, NY, USA, 2010; pp. 235–268. [Google Scholar]
- Hacking, S.A.; Boyraz, P.; Powers, B.M.; Sen-Gupta, E.; Kucharski, W.; Brown, C.A.; Cook, E.P. Surface roughness enhances the osseointegration of titanium headposts in non-human primates. J. Neurosci. Methods 2012, 211, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Dinda, G.P. Pulsed laser deposition of hydroxyapatite thin films on Ti–6Al–4V: Effect of heat treatment on structure and properties. Acta Biomater. 2009, 5, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Li, S.T.; Zhu, R.F.; Li, M.S.; Lei, T.Q. Formation of ultrafine particles in heat treated plasma-sprayed hydroxyapatite coatings. Surf. Coating Technol. 2003, 165, 65–70. [Google Scholar] [CrossRef]
- Whitehouse, D.J. Surface Characterization and Roughness Measurement in Engineering. In Photomechanics; Rastogi, P.K., Ed.; Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany, 2000; Volume 77. [Google Scholar] [CrossRef]
- Kubiak, K.J.; Wilson, M.C.T.; Mathia, T.G.; Carval, P. Wettability versus roughness of engineering surfaces. Wear 2011, 271, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Duta, L.; Popescu, A.C.; Zgura, I.; Preda, N.; Mihailescu, I.N. Wettability of Nanostructured Surfaces. In Wetting and Wettability; Aliofkhazraei, M., Ed.; Intech: Vienna, Austria, 2015. [Google Scholar] [CrossRef] [Green Version]
- US National Institute of Standards and Technology (NIST) e-Handbook of Statistical Methods. Available online: https://www.itl.nist.gov/div898/handbook (accessed on 2 November 2022).
- Sedlacek, M.; Vilhena, L.M.S.; Podgornik, B.; Vizintin, J. Surface topography modelling for reduced friction. J. Mech. Eng. 2011, 57, 674–680. [Google Scholar] [CrossRef] [Green Version]
- Stan, G.E.; Popescu, A.C.; Mihailescu, I.N.; Marcov, D.A.; Mustata, R.C.; Sima, L.E.; Petrescu, S.M.; Ianculescu, A.; Trusca, R.; Morosanu, C.O. On the bioactivity of adherent bioglass thin films synthesized by magnetron sputtering techniques. Thin Solid Films 2010, 518, 5955–5964. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Liu, H.; Yazici, H.; Ergun, C.; Webster, T.J.; Bermek, H. An in vitro evaluation of the Ca/P ratio for the cytocompatibility of nano-to-micron particulate calcium phosphates for bone regeneration. Acta Biomater. 2008, 4, 1472–1479. [Google Scholar] [CrossRef]
- León, B.; Jansen, J.A. Thin Calcium Phosphate Coatings for Medical Implants; Springer Science + Business Media: New York, NY, USA, 2009; pp. 1–328. [Google Scholar]
- Surmenev, R.A.; Surmeneva, M.A.; Evdokimov, K.E.; Pichugin, V.F.; Peitsch, T.; Epple, M. The influence of the deposition parameters on the properties of an rf-magnetron-deposited nanostructured calcium phosphate coating and a possible growth mechanism. Surf. Coat. Technol. 2011, 205, 3600–3606. [Google Scholar] [CrossRef]
- Sameer, R.P.; Zheng, C.; Wei, H.; Narendra, B.D. Wetting effects on in vitro bioactivity and in vitro biocompatibility of laser micro-textured Ca-P coating. Biofabrication 2010, 2, 025001. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Mark, A.; Pearcy, J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Okabe, Y.; Kurihara, S.; Yajima, T.; Seki, Y.; Nakamura, I.; Takano, I. Formation of super-hydrophilic surface of hydroxyapatite by ion implantation and plasma treatment. Surf. Coat. Technol. 2005, 196, 303–306. [Google Scholar] [CrossRef]
- Lotfi, M.; Nejib, M.; Naceur, M. Cell Adhesion to Biomaterials: Concept of Biocompatibility. In Advances in Biomaterials Science and Biomedical Applications; Pignatello, R., Ed.; Intech: Rijeka, Croatia, 2013; pp. 207–240. [Google Scholar] [CrossRef] [Green Version]
- Gentleman, M.M.; Gentleman, E. The role of surface free energy in osteoblast-biomaterial interactions. Int. Mater. Rev. 2014, 59, 417–429. [Google Scholar] [CrossRef]
- Pessková, V.; Kubies, D.; Hulejova, H.; Himmlova, L. The influence of implant surface properties on cell adhesion and proliferation. J. Mater. Sci. Mater. Med. 2007, 18, 465–473. [Google Scholar] [CrossRef]
- Mitra, J.; Tripathi, G.; Sharma, A.; Basu, B. Scaffolds for bone tissue engineering: Role of surface patterning on osteoblast response. RSC Adv. 2013, 3, 11073–11094. [Google Scholar] [CrossRef]
- ISO 13779-2; Implants for Surgery—Hydroxyapatite—Part 2: Coatings of Hydroxyapatite. ISO: Geneva, Switzerland, 2018.
- Popa, A.C.; Stan, G.E.; Enculescu, M.; Tanase, C.; Tulyaganov, D.U.; Ferreira, J.M.F. Superior biofunctionality of dental implant fixtures uniformly coated with durable bioglass films by magnetron sputtering. J. Mech. Behav. Biomed. Mater. 2015, 51, 313–327. [Google Scholar] [CrossRef]
- Stan, G.E.; Popa, A.C.; Galca, A.C.; Aldica, G.; Ferreira, J.M.F. Strong bonding between sputtered bioglass-ceramic films and Ti-substrate implants induced by atomic inter-diffusion post-deposition heat-treatments. Appl. Surf. Sci. 2013, 280, 530–538. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Gu, Z.; Qin, H.; Li, L.; Liu, J.; Yu, X. In vitro study on the degradation of lithium-doped hydroxyapatite for bone tissue engineering scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 185–192. [Google Scholar] [CrossRef]

















| Amplitude Parameter | Si Control | |||
|---|---|---|---|---|
| Rq [nm] | 0.31 | |||
| Rsk | −40.59 | |||
| Rku | 3606.86 | |||
| LiC | ||||
| Air | ||||
| NTT | TT400 | TT500 | TT700 | |
| Rsk | −2.11 | −1.56 | −2.27 | −6.49 |
| Rku | 10.76 | 13.26 | 13.80 | 53.05 |
| Water | ||||
| NTT | TT400 | TT500 | TT700 | |
| Rsk | −2.11 | −1.38 | −0.99 | −1.16 |
| Rku | 10.76 | 5.09 | 6.16 | 6.50 |
| LiP | ||||
| Air | ||||
| NTT | TT400 | TT500 | TT700 | |
| Rsk | −3.61 | −1.54 | −1.21 | −3.38 |
| Rku | 21.59 | 9.02 | 6.15 | 17.04 |
| Water | ||||
| NTT | TT400 | TT500 | TT700 | |
| Rsk | −3.61 | −3.98 | −3.15 | −1.28 |
| Rku | 21.59 | 20.80 | 21.28 | 5.65 |
| Element | Composition [Mean ± SD (at.%)] | |||
|---|---|---|---|---|
| LiC | ||||
| Air | ||||
| NTT | TT400 | TT500 | TT700 | |
| P | 6.7 ± 0.9 | 7.2 ± 0.6 | 6.3 ± 0.6 | 7.1 ± 0.3 |
| Ca | 11.9 ± 3.5 | 12.6 ± 1.05 | 10.8 ± 1.1 | 11.6 ± 0.7 |
| Ca/P ratio | 1.76 ± 0.3 | 1.73 ± 0.1 | 1.71 ± 0.1 | 1.64 ± 0.1 |
| Water | ||||
| NTT | TT400 | TT500 | TT700 | |
| P | 6.7 ± 0.9 | 6.4 ± 0.4 | 7.2 ± 0.8 | 7 ± 0.09 |
| Ca | 11.9 ± 3.5 | 10.7 ± 0.8 | 13.3 ± 3.4 | 11.4 ± 0.3 |
| Ca/P ratio | 1.76 ± 0.3 | 1.68 ± 0.1 | 1.85 ± 0.3 | 1.63 ± 0.1 |
| LiP | ||||
| Air | ||||
| NTT | TT400 | TT500 | TT700 | |
| P | 7.6 ± 0.07 | 6.9 ± 0.6 | 7.2 ± 0.5 | 7.1 ± 0.3 |
| Ca | 12.8 ± 0.3 | 11.2 ± 1.1 | 11.9 ± 0.8 | 11.6 ± 0.4 |
| Ca/P ratio | 1.68 ± 0.1 | 1.60 ± 0.1 | 1.65 ± 0.1 | 1.62 ± 0.1 |
| Water | ||||
| NTT | TT400 | TT500 | TT700 | |
| P | 7.6 ± 0.07 | 6.5 ± 0.3 | 7.2 ± 0.5 | 7.1 ± 0.5 |
| Ca | 12.8 ± 0.3 | 10.5 ± 0.4 | 11.9 ± 0.8 | 12.2 ± 1.01 |
| Ca/P ratio | 1.68 ± 0.1 | 1.61 ± 0.1 | 1.66 ± 0.1 | 1.71 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duta, L.; Stan, G.E.; Popescu-Pelin, G.; Zgura, I.; Anastasescu, M.; Oktar, F.N. Influence of Post-Deposition Thermal Treatments on the Morpho-Structural, and Bonding Strength Characteristics of Lithium-Doped Biological-Derived Hydroxyapatite Coatings. Coatings 2022, 12, 1883. https://doi.org/10.3390/coatings12121883
Duta L, Stan GE, Popescu-Pelin G, Zgura I, Anastasescu M, Oktar FN. Influence of Post-Deposition Thermal Treatments on the Morpho-Structural, and Bonding Strength Characteristics of Lithium-Doped Biological-Derived Hydroxyapatite Coatings. Coatings. 2022; 12(12):1883. https://doi.org/10.3390/coatings12121883
Chicago/Turabian StyleDuta, L., G. E. Stan, G. Popescu-Pelin, I. Zgura, M. Anastasescu, and F. N. Oktar. 2022. "Influence of Post-Deposition Thermal Treatments on the Morpho-Structural, and Bonding Strength Characteristics of Lithium-Doped Biological-Derived Hydroxyapatite Coatings" Coatings 12, no. 12: 1883. https://doi.org/10.3390/coatings12121883