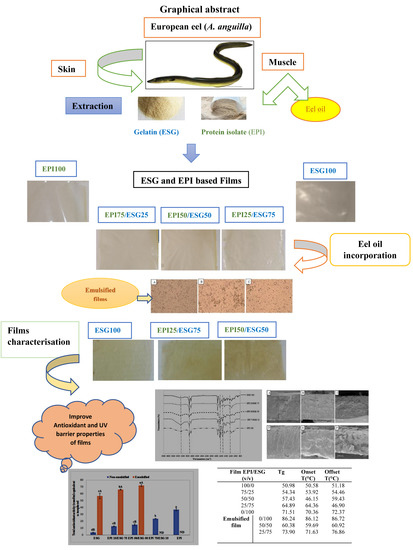
Design of Bioinspired Emulsified Composite European Eel Gelatin and Protein Isolate-Based Food Packaging Film: Thermal, Microstructural, Mechanical, and Biological Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of European Eel Protein Isolate
2.2. Preparation of European Eel Skin Gelatin
2.3. Preparation of European Eel Oil
2.4. Preparation of EPI/ESG Blend Films
2.5. Physical Characterizations of the Films
2.5.1. Moisture Content and Water Solubility
2.5.2. Color and Ultraviolet-Visible Spectroscopy (UV-Vis)
2.5.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.4. Mechanical Properties of Composite Films
2.5.5. Thermal Properties of Composite Films
2.5.6. Microstructure of Composite Films
2.6. In-Vitro Antioxidant Activity of the Films
2.7. Statistical Analysis
3. Results and Discussion
3.1. Protein Content and SDS-PAGE Profiles of EPI and ESG
3.1.1. Physicochemical Composition of EPI and ESG
3.1.2. SDS Page of ESG
3.2. Characterization of EPI/ESG Blend Films
3.2.1. Color and Light Transmission of Films
3.2.2. Moisture Content and Solubility of Films
3.2.3. FTIR Spectra
3.2.4. Mechanical Properties
3.2.5. Thermal Properties
3.2.6. Microstructure of Films
3.2.7. Antioxidant Activity of Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andrade, R.M.S.; Ferreira, M.S.L.; Gonçalves, E.C.B.A. Development and characterization of edible films Based on fruit and vegetable residues. J. Food Sci. 2016, 81, 412–418. [Google Scholar] [CrossRef]
- Chen, H.; Hu, X.; Chen, E.; Wu, S.; Mc Clements, D.J.; Liu, S.; Li, B.; Li, Y. Preparation, characterization, and properties of chitosan films with cinnamaldehyde nano emulsions. Food Hydrocoll. 2016, 61, 662–671. [Google Scholar] [CrossRef]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of edible films and coatings with antimicrobial activity. Food Bioprocess Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Romani, V.P.; Olsen, B.; Collares, M.P.; Oliveira, J.R.M.; Prentice-Hernández, C.; Martins, V.G. Improvement of fish protein films properties for food packaging through glow discharge plasma application. Food Hydrocoll. 2019, 87, 970–976. [Google Scholar] [CrossRef]
- Hamdi, M.; Nasri, R.; Li, S.; Nasri, M. Bioactive composite films with chitosan and carotenoproteins extract from blue crab shells: Biological potential and structural, thermal, and mechanical characterization. Food Hydrocoll. 2019, 89, 802–812. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B 2019, 177, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kchaou, H.; Jridi, M.; Abdelhedi, O.; Benbettaieb, N.; Karbowiak, T.; Nasri, M.; Debeaufort, F. Development and characterization of cuttlefish (Sepia officinalis) skin gelatin-protein isolate blend films. Int. J. Biol. Macromol. 2017, 105, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Bin, S.K. Preparation and Characterization of an Olive Flounder (Paralichthys olivaceus) Skin Gelatin and Polylactic Acid Bilayer Film. J. Food Sci. 2016, 82, 706–710. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Songtipya, P. Characteristics of film based on protein isolate from red tilapia muscle with negligible yellow discoloration. Int. J. Biol. Macromol. 2011, 48, 758–767. [Google Scholar] [CrossRef]
- Paschoalick, T.M.; Garcia, F.T.; Sobral, P.J.A.; Habitante, A.M.Q.B. Characterisation of some functional properties of edible films based on muscle proteins of Nile tilapia. Food Hydrocoll. 2003, 17, 419–427. [Google Scholar] [CrossRef]
- Rocha, M.D.; Loiko, M.R.; Gautério, G.V.; Tondo, E.C.; Prentice, C. Influence of heating, protein and glycerol concentrations of film-forming solution on the film properties of Argentine anchovy (Engraulis anchoita) protein isolate. J. Food Eng. 2013, 116, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Mansha, M.; Mazumder, M.A.J.; Jafar Mazumder, M.A. Functional Polymers, 1st ed.; Springer: Berlin, Germany, 2018. [Google Scholar]
- Kokoszka, S.; Debeaufort, F.; Lenart, A.; Voilley, A. Liquid and vapor water transfer through whey protein/lipid emulsion films. J. Sci. Food Agric. 2010, 90, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Arab-Tehrany, E.; Jacquot, M.; Gaiani, C.; Imran, M.; Desobry, S.; Linder, M. Beneficial effects and oxidative stability of omega-3 long-chain polyunsaturated fatty acids. Trends Food Sci. Technol. 2012, 25, 24–33. [Google Scholar] [CrossRef]
- Limpisophon, K.; Tanaka, M.; Osako, K. Characterization of gelatin-fatty acid emulsion films based on blue shark (Prionace glauca) skin gelatin. Food Chem. 2010, 122, 1095–1101. [Google Scholar] [CrossRef]
- Taktak, W.; Nasri, R.; Hamdi, M.; Gomez-Mascaraque, L.G.; Lopez-Rubio, A.; Li, S.; Nasri, M.; Chaâbouni-Karra, M. Physicochemical, textural, rheological and microstructural properties of protein isolate gels produced from European eel (Anguilla anguilla) by heat-induced gelation process. Food Hydrocoll. 2018, 82, 278–287. [Google Scholar] [CrossRef]
- Taktak, W.; Nasri, R.; Lopez-Rubio, A.; Hamdi, M.; Gomez-Mascaraque, L.G.; Ben Amor, N.; Kabadou, A.; Li, S.; Nasri, M.; Chaâbouni-Karra, M. Improved antioxidant activity and oxidative stability of spray dried European eel (Anguilla anguilla) oil microcapsules: Effect of emulsification process and eel protein isolate concentration. Mater. Sci. Eng. C 2019, 104, 109867. [Google Scholar] [CrossRef]
- Jridi, M.; Nasri, R.; Lassoued, I.; Souissi, N.; Mbarek, A.; Barkia, A.; Nasri, M. Chemical and biophysical properties of gelatins extracted from alkali-pretreated skin of cuttlefish (Sepia officinalis) using pepsin. Food Res. Int. 2013, 54, 1680–1687. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Gennadios, A.; Handa, A.; Froning, G.W.; Weller, C.L.; Hanna, M.A. Physical properties of egg white-dialdehyde starch films. J. Agric. Food. Chem. 1998, 46, 1297–1302. [Google Scholar] [CrossRef]
- ISO 527-3:1995. Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets; ISO: Geneva, Switzerland, 1995. [Google Scholar]
- Yildirim, A.; Mavi, A.; Oktay, M.; Kara, A.A.; Algur, O.F.; Bilaloglu, V. Comparison of antioxidant and antimicrobial activities of tilia (Tilia arentea Desf Ex DC) sage (Salvia triloba L.) and black tea (Camellia sinensis L.) extracts. J. Agric. Food. Chem. 2001, 48, 5030–5034. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food. Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphor molybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Skin gelatin from bigeye snapper and brownstripe red snapper: Chemical compositions and effect of microbial transglutaminase on gel properties. Food Hydrocoll. 2006, 20, 1216–1222. [Google Scholar] [CrossRef]
- Monograph, G.M.E. Standard Methods for the Testing of Edible Gelatin; Europe (GME) Monograph: Brussels, Belgium, 2000. [Google Scholar]
- Duan, R.; Zhang, J.; Liu, L.; Cui, L.; Regensteinhe, M.J. Functional properties and application of gelatin derived from the skin of channel catfish (Ictalurus punctatus). Food Chem. 2018, 239, 464–469. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Badii, F.; Howell, N.K. The effect of chicken skin gelatin and whey protein interactions on rheological and thermal properties. Food Hydrocoll. 2015, 45, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Nilsuwan, K. Emulsion film based on fish skin gelatin and palm oil: Physical, structural and thermal properties. Food Hydrocoll. 2015, 48, 248–259. [Google Scholar] [CrossRef]
- Iwata, K.; Ishizaki, S.; Handa, A.; Tanaka, M. Preparation and characterization of edible films from fish water-soluble proteins. Fish. Sci. 2000, 66, 372–378. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, C.H.; Wen, Q.B.; Li, L.; Yang, X.Q. Effect of processing parameters on the properties of transglutaminase-treated soy protein isolate films. Innovative Food Sci. Emerg. Technol. 2007, 8, 218–225. [Google Scholar] [CrossRef]
- Zhang, Y.; Simpson, B.K.; Dumont, M.J. Effect of beeswax and carnauba wax addition on properties of gelatin films: A comparative study. Food Biosci. 2018, 26, 88–95. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Osako, K. Development and characterization of blend films based on fish protein isolate and fish skin gelatin. Food Hydrocoll. 2014, 39, 58–67. [Google Scholar] [CrossRef]
- Chinabhark, K.; Benjakul, S.; Prodpran, T. Effect of pH on the properties of protein-based film from bigeye snapper (Priacanthustayenus) surimi. Bioresour. Technol. 2007, 98, 221–225. [Google Scholar] [CrossRef]
- Denavi, G.A.; Perez-Mateos, M.; Anon, M.C.; Montero, P.; Mauri, A.N.; Guillen, M.C.G. Structural and functional properties of soy protein isolate and cod gelatin blend films. Food Hydrocoll. 2009, 23, 2094–2101. [Google Scholar] [CrossRef] [Green Version]
- Hoque, M.S.; Benjakul, S.; Prodpran, T.; Songtipya, P. Properties of blend film based on cuttlefish (Sepia pharaonis) skin gelatin and mung bean protein isolate. Int. J. Biol. Macromol. 2011, 49, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Burger, T.G.; Zhang, Y. Recent Progress in the Utilization of Pea Protein as an Emulsifier for Food Applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Monedero, F.M.; Hambleton, A.; Talens, P.; Debeaufort, F.; Chiralt, A.; Voilley, A. Study of the retention and release of n-hexanal incorporated into soy protein isolate-lipid composite films. J. Food Eng. 2010, 100, 133–138. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Beatty, E.; Roos, Y.H.; Morris, M.A.; Kerry, J.P. Development and characterization of biodegradable composite films based on gelatin derived from beef, pork and fish sources. Foods 2013, 2, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Muyonga, J.; Cole, C.; Duodu, K. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Latesniloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Pérez-Mateos, M.; Gómez-Estaca, J.; López- Caballero, E.; Giménez, B.; Montero, P. Fish gelatin: A renewable material for developing active biodegradable films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Moomand, K.; Lim, L.T. Oxidative stability of encapsulated fish oil in electrospun zein fibres. Food Res. Int. 2014, 62, 523–532. [Google Scholar] [CrossRef]
- Córdoba, L.J.P.; Sobral, P.J.A. Physical and antioxidant properties of films based on gelatin, gelatin-chitosan or gelatin-sodium caseinate blends loaded with nanoemulsified active compounds. J. Food Eng. 2017, 213, 47–53. [Google Scholar] [CrossRef]
- Guillen, M.D.; Cabo, N. Usefulness of the frequency data of the fourier transform infrared spectra to evaluate the degree of oxidation of edible oils. J. Agric. Food. Chem. 1999, 47, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. J. Food Chem. 2016, 194, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Souissi, N.; Abdelhedi, O.; Mbarek, A.; Kammoun, W.; Kchaou, H.; Nasri, M. Gelatin based bio-films prepared from grey triggerfish’ skin influenced by enzymatic pretreatment. Int. J. Biol. Macromol. 2017, 105, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Abdelhedi, O.; Nasri, R.; Souissi, N.; Nasri, M.; Jridi, M. Sulfated polysaccharides from common smooth hound: Extraction and assessment of anti-ACE, antioxidant and antibacterial activities. Carbohydr. Polym. 2016, 152, 605–614. [Google Scholar] [CrossRef]
- Tang, C.H.; Xiao, M.L.; Chen, Z.; Yang, X.Q.; Yin, S.W. Properties of cast films of vicilin-rich protein isolates from Phaseolus legumes: Influence of heat curing. LWT Food Sci. Technol. 2009, 42, 1659–1666. [Google Scholar] [CrossRef]
- Akköse, A. Effect of Various Biopolymers on Glass Transition Temperature of Chicken Breast Meat. Akademik Gıda 2018, 16, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Arfat, Y.A.; Ahmed, J.; Hiremath, N.; Auras, R.; Joseph, A. Thermo-mechanical, rheological, structural and antimicrobial properties of bionanocomposite films based on fish skin gelatin and silver-copper nanoparticles. Food Hydrocoll. 2017, 62, 191–202. [Google Scholar] [CrossRef]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of cross-linking with calcium ions on the physical properties of alginate films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar] [CrossRef]
- Levine, H.; Slade, L. A polymer physicochemical approach to the study of commercial starch hydrolysis products (SHPs). Carbohydr. Polym. 1986, 6, 213–244. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Chai, Z.; Leng, X. Study of the physical properties of whey protein isolate and gelatin composite films. J. Agric. Food. Chem. 2010, 58, 5100–5108. [Google Scholar] [CrossRef] [PubMed]
- Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Caseinate films modified with tung oil. Food Hydrocoll. 2010, 24, 800–808. [Google Scholar] [CrossRef]
- Butnaru, E.; Stoleru, E.; Brebu, M.A.; Darie-Nita, R.N.; Bargan, A.; Vasile, C. Chitosan-Based Bionanocomposite Films Prepared by Emulsion Technique for Food Preservation. Materials 2019, 12, 373. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Ghosh, U. Natural antioxidants-The key to safe and sustainable life. Int. J. Latest Trends Eng. Tech. 2016, 6, 201. [Google Scholar]
- Khantaphant, S.; Benjakul, S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatine hydrolysate with antioxidative activity. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 410–419. [Google Scholar] [CrossRef]
- Bao, S.; Xu, S.; Wang, Z. Antioxidant activity and properties of gelatin films incorporated with tea polyphenol-loaded chitosan nanoparticles. J. Sci. Food Agr. 2009, 89, 2692–2700. [Google Scholar] [CrossRef]
- Giménez, B.; Gómez-Estaca, J.; Alemán, A.; Gómez-Guillén, M.C.; Montero, M.P. Improvement of the antioxidant properties of squid skin gelatin films by the addition of hydrolysates from squid gelatin. Food Hydrocoll. 2009, 23, 1322–1327. [Google Scholar] [CrossRef] [Green Version]
- Jouki, M.; Mortazavi, S.A.; Tabatabaee, F.; Koocheki, A. Characterization of antioxidant–antibacterial quince seed mucilage films containing thyme essential oil. Carbohydr. Polym. 2013, 99, 537–546. [Google Scholar] [CrossRef]







| Samples | Humidity (%) | Protein (%) | Lipid (%) | Ash (%) |
|---|---|---|---|---|
| ES | 58 ± 2.25 a | 30.72 ± 0.94 b | 6.7 ± 0.86 a | ± 0.24 a |
| ESG | 6.10 ± 0.14 b | 90.5 ± 0.70 a | 1.12± 0.21 b | 2.95 ± 0.07 a |
| Films EPI/ESG (w/w) | Color Parameter | ΔE | |||
|---|---|---|---|---|---|
| L | a | b | |||
| Not emulsified | 100/0 | 85.55 ± 0.37 a | −0.67 ±0.05 a | 7.93 ± 0.95 c | – |
| 75/25 | 84.24 ± 0.73 ab | −0.61 ± 0.06 a | 8.15 ± 0.43 c | 1.33 ± 0.78 c | |
| 50/50 | 83.09 ± 0.58 bcA | −0.58 ± 0.01 aB | 13.32 ± 1.07 bB | 5.93 ± 1.21 bB | |
| 25/75 | 81.64 ± 0.71 cdA | −0.61 ± 0.08 aB | 13.35 ± 0.01 bB | 6.69 ± 0.40 bB | |
| 0/100 | 80.91 ± 1.18 dA | −0.55 ± 0.02 aB | 17.06 ± 1.62 aB | 8.78 ± 0.90 aB | |
| Emulsified | 0/100 | 79.36 ± 0.25 bA | 0.91 ± 0.16 aA | 20.69 ± 0.09 aA | 14.27 ± 1.01 aA |
| 25/75 | 80.55 ± 0.09 aA | 0.41 ± 0.05 bA | 18.83 ± 0.01 bA | 12.04 ±1.02 abA | |
| 50/50 | 81.58 ± 0.50 aA | 0.45 ± 0.09 bA | 17.56 ± 0.38 cA | 10.48±1.02 bA | |
| Films EPI/ESG (w/w) | Light Transmittance (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 | 280 | 350 | 400 | 500 | 600 | 700 | 800 | ||
| Not Emulsified | 100/0 | 0.1 | 0.06 | 15.82 | 29.24 | 46.22 | 52.88 | 57.76 | 60.60 |
| 75/25 | 0.1 | 0.27 | 19.34 | 33.15 | 47.86 | 53.09 | 57.01 | 59.47 | |
| 50/50 | 0.08 | 0.14 | 11.43 | 20.71 | 31.57 | 35.68 | 38.65 | 40.31 | |
| 25/75 | 0.08 | 0.05 | 1.50 | 3.73 | 7.77 | 10.30 | 12.30 | 13.80 | |
| 0/100 | 0.03 | 0.04 | 0.56 | 1.34 | 3.01 | 4.32 | 5.57 | 6.62 | |
| Emulsified | 0/100 | 0.05 | 0.04 | 0.58 | 1.81 | 4.40 | 6.33 | 8.10 | 9.50 |
| 25/75 | 0.05 | 0.05 | 1.63 | 4.19 | 8.72 | 11.02 | 12.54 | 13.56 | |
| 50/50 | 0.06 | 0.08 | 0.51 | 1.38 | 3.12 | 4.04 | 4.68 | 5.11 | |
| Films EPI/ESG (w/w) | Thickness (µm) | TS (MPa) | EAB (%) | Moisture Content (g moisture/100 g film) | Film Solubility (%) | |
|---|---|---|---|---|---|---|
| Not Emulsified | 100/0 | 32.00 ± 4.32 b | 5.42 ± 0.69 c | 2.14 ± 0.02 d | 6.78 ± 0.40 d | 29.47 ± 1.03 e |
| 75/25 | 50.78 ± 3.03 a | 10.69 ± 1.20 b | 8.55 ± 0.61 c | 7.58 ± 0.24 c | 41.91 ± 1.86 d | |
| 50/50 | 52.60 ± 2.41 a | 14.33 ± 0.73 a | 15.00 ± 0.68 b | 7.94 ± 0.35 cA | 64.99 ± 2.07 cB | |
| 25/75 | 56.25 ± 4.79 a | 16.34 ± 0.30 a | 16.05 ± 0.62 b | 8.87 ± 0.03 bB | 72.43 ± 0.60 bB | |
| 0/100 | 59.00 ± 2.00 a | 16.58 ± 0.32 a | 18.74 ± 0.74 a | 12.26 ± 0.10 aA | 93.01 ± 0.54 aB | |
| Emulsified | 0/100 | ND | ND | ND | 10.12 ± 0.60 aB | 96.51 ± 2.06 aA |
| 25/75 | ND | ND | ND | 10.36 ± 0.99 aA | 91.64 ± 0.39 bA | |
| 50/50 | ND | ND | ND | 8.36 ± 1.35 aA | 87.06 ± 0.99 cA | |
| Film EPI/ESG (w/w) | Tg | Onset T (°C) | Offset T (°C) | |
|---|---|---|---|---|
| Not Emulsified | 100/0 | 50.98 | 50.58 | 51.18 |
| 75/25 | 54.34 | 53.92 | 54.46 | |
| 50/50 | 57.43 | 46.15 | 59.43 | |
| 25/75 | 64.89 | 64.36 | 46.90 | |
| 0/100 | 71.51 | 70.36 | 72.37 | |
| Emulsified | 0/100 | 86.24 | 86.12 | 86.72 |
| 25/75 | 73.90 | 59.69 | 60.92 | |
| 50/50 | 60.38 | 71.63 | 76.86 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taktak, W.; Kchaou, H.; Hamdi, M.; Li, S.; Nasri, M.; Karra-Chaâbouni, M.; Nasri, R. Design of Bioinspired Emulsified Composite European Eel Gelatin and Protein Isolate-Based Food Packaging Film: Thermal, Microstructural, Mechanical, and Biological Features. Coatings 2020, 10, 26. https://doi.org/10.3390/coatings10010026
Taktak W, Kchaou H, Hamdi M, Li S, Nasri M, Karra-Chaâbouni M, Nasri R. Design of Bioinspired Emulsified Composite European Eel Gelatin and Protein Isolate-Based Food Packaging Film: Thermal, Microstructural, Mechanical, and Biological Features. Coatings. 2020; 10(1):26. https://doi.org/10.3390/coatings10010026
Chicago/Turabian StyleTaktak, Wafa, Hela Kchaou, Marwa Hamdi, Suming Li, Moncef Nasri, Maha Karra-Chaâbouni, and Rim Nasri. 2020. "Design of Bioinspired Emulsified Composite European Eel Gelatin and Protein Isolate-Based Food Packaging Film: Thermal, Microstructural, Mechanical, and Biological Features" Coatings 10, no. 1: 26. https://doi.org/10.3390/coatings10010026